Genes & Cancer
Ectopic expression of lncRNA MVIH as a potential diagnostic biomarker in cervical cancer
Mohammad Ghanbari1, Aida Aghazadeh1, Elaheh Malekabbaslou1, Ali Rajabi1, Aref Sobhkhizy2, Melika Maydanchi2, Ali Saber2 and Reza Safaralizadeh1
1 Department of Animal Biology, Faculty of Natural Sciences, University of Tabriz, Tabriz, Iran
2 Zimagene Medical Genetics Laboratory, Hamedan, Iran
Correspondence to: Reza Safaralizadeh, email: [email protected]
Keywords: MVIH; lncRNA; non-coding RNA; biomarker; cervical cancer
Received: April 25, 2022
Accepted: November 18, 2022
Published: November 23, 2022
Copyright: © 2022 Ghanbari et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ABSTRACT
Aim: Cervical cancer (CC) is one of the most common cancers in women. Recent advances in screening and vaccination against the papilloma virus (HPV) have increased protection against CC. However, there is no effective diagnostic biomarker and treatment approach during the course of the disease. The current study is thus aimed to evaluate the changes in the expression of lncRNA associated with microvascular invasion in hepatocellular carcinoma (lncRNA MVIH) and its diagnostic value as a biomarker in CC patients.
Materials and Methods: One-hundred and fifteen (n = 115) pairs of CC primary tumor and marginal non-tumor tissue samples were obtained from Tabriz Valiasr International Hospital (Tabriz, Iran). RNA extraction and cDNA synthesis followed by quantitative reverse transcriptase PCR (qRT-PCR) were considered to investigate alterations in the expression levels of MVIH in patients with CC. The associations between MVIH expression changes and clinicopathological features as well as its potential as a diagnostic biomarker were assessed using SPSS and GraphPad prism software and the receiver operating characteristic (ROC).
Results: The expression levels of MVIH were significantly higher in CC tumors as compared to marginal non-tumor samples (p < 0.0001). Overexpression of MVIH was significantly associated with younger age (p = 0.033), lymph node metastasis (p = 0.031), tumor invasion depth (p = 0.035), and squamous cell type of CC (p = 0.019). The ROC analysis for MVIH as a diagnostic biomarker revealed the respective sensitivity and specificity of 67.83 and 80.
Conclusions: Overexpression of MVIH in CC tumors suggests its oncogenic role during tumorigenesis. Thus, it may serve as a potential diagnostic biomarker.
INTRODUCTION
CC is the second most common cancer and the fourth malignant cause of death in women worldwide [1]. Infection with HPV is the most important risk factor for CC. Other factors such as multiple sexual partners, marriage before the age of 18, smoking, and the use of oral contraceptive pills can raise the risk of CC. However, genetic susceptibility has been estimated to be lower than 1% [1, 2]. The origin of CC is the epithelium of the uterine cervix, particularly the squamocolumnar junction of the ectocervix and endocervix. Histologically, CC is divided into two main groups including squamous cell carcinoma (95%) and adenocarcinoma (5%) [3]. CC can be largely preventable by regular screening methods such as histologically checking epithelial cells and HPV testing on samples obtained by pap smears [4]. In addition, vaccination against HPV can significantly decrease the incidence of CC [5]. Radiotherapy and chemotherapy are the main treatments in patients with CC. However, these treatments are not always effective as they are associated with severe side effects and relapse [6]. Also, due to lack of suitable diagnostic biomarkers during CC development, it has progressed to invasive stages in most of the patients, which has resulted in lower survival rate [7]. So far, most biomarkers that have been used in clinically diagnosis in CC, are proteins such as squamous cell carcinoma antigen (SCC), serum fragments of cytokeratin 19 (CYFRA 21-1), and cancer antigen-125 (CA 125). However, the specificity and sensitivity of these biomarkers in early detection of CC are low [8]. Therefore, the identification of novel molecular biomarkers and therapeutic targets could be of great importance to improve clinical outcomes in patients with CC. In recent years, lncRNAs have received more attention in cancer research because of their tissue specificity as well as their role in tumorigenesis [9].
lncRNAs are more than 200 nucleotides long and do not encode proteins. They regulate the expression of critical genes through several biological processes at epigenetic, transcription, and post transcription levels [9]. The expression pattern of lncRNAs are more specific in cells and tissues than mRNAs and also, lncRNAs are easily found in body fluids due to their stability [10, 11]. Furthermore, lncRNAs have critical roles in regulating genes involved in cell cycle, apoptosis, angiogenesis, and metastasis [12]. All of these features make lncRNAs suitable biomarkers of diagnostic and prognostic biomarkers in many cancers so that dysregulation of lncRNAs in tumor tissues is associated with tumor type and stage. For example, lncRNAs such as HOXA-AS2, FOXD2-AS, and KRT18P55 are upregulated in gastric cancer (GC) and they are significantly associated with clinical parameters such as tumor size, lymph node metastasis and H. pillory infection, introducing them potential tumor markers for GC [13–15]. In addition, lncRNAs such as HOTAIR, H19, and MALAT1 have oncogenic roles since their upregulation in CC tumor cells leads to excessive cell proliferation and migration [16–18]. Moreover, other lncRNAs such as lncRNA MEG3 and lncRNA GAS5 have tumor-suppressing roles and they are downregulated during the development of CC [19, 20]. Analysis of diagnostic and prognostic value of lncRNA MEG3 showed it can be a potential biomarker in CC [21]. Also, downregulation of GAS5 predicts poor prognosis of patients with CC [22]. These studies show lncRNAs by having oncogenic and tumor suppressor roles, may be used as diagnostic and prognostic biomarkers to improve clinical outcomes in patients with CC, therefore detection of new lncRNAs as biomarkers of CC is valuable in diagnosis of CC in early stages [9].
lncRNA MVIH was first identified in hepatocellular carcinoma (HC). Its overexpression is associated with a poor prognosis, increased angiogenesis, and HC aggressiveness [23]. Moreover, dysregulation of MVIH can serve as a prognostic biomarker in non-small cell lung cancer (NSCLC) and breast cancer [24, 25]. However, the expression pattern of MVIH in CC has not been fully elucidated. This research is aimed to evaluate the expression levels of MVIH in CC tumors to assess the potential of this lncRNA as a diagnostic biomarker in CC patients.
RESULTS
Patient characteristics
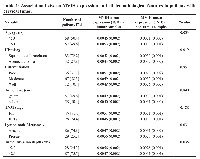
One-hundred and fifteen (115) consecutive patients with CC were included in this study. The mean age of the patients was 50.93 (SD ± 10.20 years). Fifty-five (49.6%) patients were younger than 50 years old while the remaining (50.4%) were older than 50. Eighty-three (72.2%) patients had squamous cell carcinoma and 32 (27.8%) had adenocarcinoma subtype. Approximately, 31.3% of patients had poorly differentiated tumors, followed by 58.3% with moderate and 10.4% with well-differentiated tumors. The invasion depth of the tumor in the cervix was more than 2.3 cm in 28 (24.4%) patients and the remaining (75.6%) had a depth lower than 2.3 cm. In 57 (49.6%) patients, the size of the tumor was more than 5 cm. Approximately, 25.2% (29) of patients had lymph node metastasis. According to the TNM staging, 87 (75.6%) patients were in stage I/II, whereas 28 (24.4%) cases were in stage III/IV (Table 1).
The expression of MVIH in CC tumor
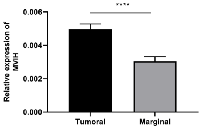
qRT-PCR was performed to assess the expression of MVIH in CC primary tumors relative to marginal non-tumor tissue. Our results revealed that the expression of MVIH was significantly higher in the tumor as compared to non-tumor tissues (p < 0.0001) (Figure 1).
Association between MVIH expression levels and clinicopathological features
The overexpression of MVIH was significantly associated with the patients’ age (younger than 50 years old) (p = 0.033). A significant positive relationship was detected between upregulation of MVIH and lymph node involvement (p = 0.031). The expression of MVIH was significantly higher in tumors with deeper invasion into the cervix (p = 0.035). Furthermore, MVIH was significantly overexpressed in the squamous cell subtype compared to the adenocarcinoma subtype (p = 0.019). No significant association was observed between elevated levels of MVIH and other clinicopathological features such as tumor size, tumor differentiation, and disease stage (Table 1).
MVIH expression as a potential biomarker of CC
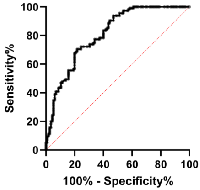
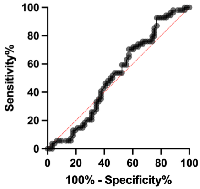
The ROC analysis was employed to examine MVIH potentials as a diagnostic biomarker for CC. Our analysis revealed a sensitivity and specificity of 67.83 and 80, respectively. The area under curve (AUC) was 0.8114 and the cut-off value was set to 0.0039 (Figure 2 and Table 2). Also, the ROC analysis of distant metastasis revealed MVIH as a weak prognostic biomarker with sensitivity and specificity of 53.70 and 52.46 respectively. The AUC was 0.5213 and the cut-off value was <0.004804 (Figure 3 and Table 3).
DISCUSSION
In the present study, lncRNA MVIH expression levels were assessed in CC tumors as compared to marginal non-tumor samples. MVIH was significantly upregulated in CC tumor tissue. Furthermore, overexpression of MVIH was significantly associated with younger age, squamous cell subtype, lymph node metastasis, and tumor depth of invasion.
The overexpression of MVIH was found in CC tumors in comparison with non-tumor tissues which is consistent with previous studies on other types of cancer, supporting the oncogenic role of this lncRNA in cancer. MVIH imposes an oncogenic mechanism by stimulation of several pathways with crucial roles in angiogenesis, cell proliferation, invasion, and apoptosis resistance [23]. However, the answer to the question of how MVIH plays such an oncogenic role in CC tumorigenesis requires deeper functional studies.
Our finding revealed a significant association between MVIH overexpression and depth of invasion and lymph node metastasis which is consistent with findings in NSCLC and glioblastoma; where MVIH overexpression is correlated with metastasis and tumor invasiveness. MVIH upregulation promotes invasion of NSCLC cells via matrix metalloproteinase 2 and 9 (MMP2/MMP9) expression in vitro [24]. In glioma, the overexpression of MVIH was associated with cancer cell proliferation, invasion, and migration [26]. In addition, MVIH is involved in the upregulation of AKT and CXCR4 by sponging miR-302a leading to cell proliferation and invasion in glioblastoma [27]. MVIH also plays a decisive role in cell proliferation, angiogenic, and anti-apoptotic pathways. Yuan et al. (2012) showed that MVIH expression leads to angiogenesis in HC via inhibition of an anti-angiogenic protein known as PGK1 [23]. On the other hand, Shi et al. (2015) revealed that MVIH inhibits apoptosis in HC cells and stimulates cell growth through modulating miR-199a expression [28].
Overexpression of MVIH in breast cancer and acute myeloid leukemia (AML) cells resulted in increased cell proliferation and inhibited apoptosis, while MVIH knockdown suppressed cell proliferation and enhanced apoptosis [25, 29]. In addition, the expression of MVIH is correlated with Ki67 expression in breast cancer. The expression of nuclear antigen Ki67 is a proliferative index of cycling cells [25, 30, 31]. Another study revealed that MVIH overexpression led to increased cell proliferation and resistance to apoptosis in AML cells via sponging miR-505. In this mechanism, by blocking miR-505, MVIH triggers the upregulation of oncogenic genes including HMGB1 and CCNE2 which have important roles in cell proliferation and resistance to apoptosis [32]. Despite extensive studies on different cancer types, angiogenic and anti-apoptotic roles of MVIH in CC have not been clarified and more precise functional studies are required. However, the mediatory role of MVIH in cancer cell invasiveness makes it a potential therapeutic target in cancer therapy.
lncRNAs are strictly regulated in cells and show cell type and tissue specifications. They play important roles in diverse biological processes and their dysregulation has been reported in a wide range of malignancies, suggesting them as high-potential biomarkers [33]. lncRNAs could be suitable biomarkers because they are stable, tissue-specific, and easily detectable in body fluids [34]. Several studies have shown the prognostic potential of MVIH as a marker in different cancer types such as AML, gastric, glioma, and breast cancers [26, 29, 35, 36]. Similarly, this study indicated that MVIH can be used as a potential diagnostic biomarker in CC patients. However, it seems that MVIH could be a weak prognostic biomarker in metastasis to lymph node.
MATERIALS AND METHODS
Tissue specimens
One hundred and fifteen pairs of CC tissue and marginal non-tumor tissue samples were collected from Tabriz Valiasr International Hospital (Tabriz, Iran). The Medical Ethics Committee of the University of Tabriz approved the study (approval number: IR. TABRIZU. REC. 1398.015). Written informed consent was obtained from patients. This study was conducted in compliance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. Tissue specimens were collected and immediately placed in liquid nitrogen. All frozen samples were stored at −80°C. An experienced pathologist examined and determined the histopathological characteristics of the specimens.
RNA isolation and cDNA synthesis
Total RNA was isolated from samples using TRIZOL reagent based on the manufacturer’s protocol (Invitrogen, Waltham, MA, USA). RNA samples were treated with DNaseI (GeneAll, Seoul, Korea) to remove DNA contamination. RNA was quantitatively and qualitatively examined using a nanodrop (Thermo Fisher Scientific, Waltham, MA, USA) and on 3% (w/v) agarose gel electrophoresis, respectively.
Approximately, 500 ng total RNA was used as the template for cDNA synthesis using the Takara cDNA synthesis kit (Takara, Kusatsu, Japan) according to the manufacturer’s instructions. All RNA and cDNA samples were stored at −80°C.
qRT-PCR
PCR primers are listed in Table 4. qRT-PCR was performed using SYBR Green Master Mix (Amplicon, Odense, Denmark) and a Light Cycler® 96 Real-Time PCR system (Roche Molecular Systems, Pleasanton, CA, USA). The total volume of each reaction was 14 μl comprising 7 μl of SYBR Green Master Mix (2×), 0.6 μl of specific primers for MVIH (10 μM) and β-actin (10 μM), 1 μl of cDNA (100 ng/μl), and 5.4 μl ddH2O. The thermal cycling program was as follows: step 1: 95°C for 10 min, step 2: 40 cycles including 95°C for 30 sec and 60°C for 30 sec, and 72°C for 30 sec, and step 3: 72°C for 5 min. Each experiment was carried out in triplicate and relative MVIH expression was evaluated using the comparative cycle threshold (Ct) method. MVIH gene expression levels were then normalized to β-actin expression levels and the difference between MVIH and β-actin Ct values (ΔCt) was calculated for each sample. Finally, MVIH expression levels were determined in tumor versus non-tumor tissues by calculating 2−ΔCt.
Statistical analysis
SPSS version 26 software (SPSS Inc., Chicago, IL, USA) and GraphPad Prism Version 9.0 (GraphPad software, San Diego, CA, USA) were used for statistical analysis. Mann-Whitney and one-way ANOVA tests were used to analyze the association between MVIH expression levels and clinicopathological features. The ROC analysis was also conducted to determine the sensitivity and specificity of MVIH as a biomarker in patients with CC. The confidence interval (CI) was 95% and p-values smaller than 0.05 were considered statistically significant.
CONCLUSIONS
In conclusion, MVIH is significantly overexpressed in CC tumors in comparison with non-tumor tissues. The overexpression of MVIH is associated with tumor invasion depth and lymph node metastasis, it can be also considered a potential diagnostic biomarker in patients with CC to improve their clinical outcomes.
Author contributions
AR, AS, and RS designed the study. MG, AA, EM, AR, AF, and MM performed experimental works. MG, AA, and AR analyzed the data. MG wrote the manuscript with significant input from AS, AR, RS, and MM. AS edited the final draft and provided technical advice. RS supervised the project. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST
Authors have no conflicts of interest to declare.
Ethical statement
Medical Ethics Committee of the University of Tabriz approved the study (approval number: IR. TABRIZU. REC. 1398.015).
Consent
Written informed consent was obtained from patients.
- 1. Cervical cancer: Epidemiology, risk factors and screening. Chin J Cancer Res. 2020; 32:720–28. https://doi.org/10.21147/j.issn.1000-9604.2020.06.05. [Pubmed]
- 2. Risk Factors of Cervical Cancer: A Case-Control Study. Asia Pac J Oncol Nurs. 2019; 6:308–14. https://doi.org/10.4103/apjon.apjon_73_18. [Pubmed]
- 3. Histopathology of cervical precursor lesions and cancer. Acta Dermatovenerol Alp Pannonica Adriat. 2011; 20:125–33. [Pubmed]
- 4. A Study on Cervical Cancer Screening Using Pap Smear Test and Clinical Correlation. Asia Pac J Oncol Nurs. 2018; 5:337–41. https://doi.org/10.4103/apjon.apjon_15_18. [Pubmed]
- 5. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020; 395:575–90. https://doi.org/10.1016/S0140-6736(20)30068-4. [Pubmed]
- 6. Cervical cancer: Biomarkers for diagnosis and treatment. Clin Chim Acta. 2015; 445:7–11. https://doi.org/10.1016/j.cca.2015.03.005. [Pubmed]
- 7. Impact of provider-patient communication on cancer screening adherence: A systematic review. Prev Med. 2016; 93:96–105. https://doi.org/10.1016/j.ypmed.2016.09.034. [Pubmed]
- 8. Serum biomarkers for early detection of gynecologic cancers. Cancers (Basel). 2010; 2:1312–27. https://doi.org/10.3390/cancers2021312. [Pubmed]
- 9. Molecular mechanisms of long noncoding RNAs and their role in disease pathogenesis. Oncotarget. 2018; 9:18648–63. https://doi.org/10.18632/oncotarget.24307. [Pubmed]
- 10. Tissue Expression Difference between mRNAs and lncRNAs. Int J Mol Sci. 2018; 19:3416. https://doi.org/10.3390/ijms19113416. [Pubmed]
- 11. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013; 113:1–11. https://doi.org/10.1007/s11060-013-1084-8. [Pubmed]
- 12. Roles and Mechanisms of the Long Noncoding RNAs in Cervical Cancer. Int J Mol Sci. 2020; 21:9742. https://doi.org/10.3390/ijms21249742. [Pubmed]
- 13. Overexpression of HOXA-AS2 LncRNA in Patients with Gastric Cancer and Its Association with Helicobacter pylori Infection. J Gastrointest Cancer. 2022; 53:72–77. https://doi.org/10.1007/s12029-020-00549-y. [Pubmed]
- 14. Moderate Prognostic Value of lncRNA FOXD2-AS1 in Gastric Cancer with Helicobacter pylori Infection. J Gastrointest Cancer. 2022; 53:687–91. https://doi.org/10.1007/s12029-021-00686-y. [Pubmed]
- 15. Prognostic Value of LncRNA KRT18P55 in Patients with Intestinal Type of Gastric Cancer. J Gastrointest Cancer. 2022; 53:1014–19. https://doi.org/10.1007/s12029-021-00744-5. [Pubmed]
- 16. HOTAIR contributes to cell proliferation and metastasis of cervical cancer via targetting miR-23b/MAPK1 axis. Biosci Rep. 2018; 38:BSR20171563. https://doi.org/10.1042/BSR20171563. [Pubmed]. Retraction in: Biosci Rep. 2021; 41:BSR-2017-1563_RET. https://doi.org/10.1042/BSR-2017-1563_RET. [Pubmed]
- 17. Decreased Expression of miR-138-5p by lncRNA H19 in Cervical Cancer Promotes Tumor Proliferation. Oncol Res. 2018; 26:401–10. https://doi.org/10.3727/096504017X15017209042610. [Pubmed]
- 18. High MALAT1 expression predicts a poor prognosis of cervical cancer and promotes cancer cell growth and invasion. Eur Rev Med Pharmacol Sci. 2015; 19:3187–93. [Pubmed]
- 19. Long non-coding RNA MEG3 inhibits cervical cancer cell growth by promoting degradation of P-STAT3 protein via ubiquitination. Cancer Cell Int. 2019; 19:175. https://doi.org/10.1186/s12935-019-0893-z. [Pubmed]
- 20. LncRNA GAS5 suppresses the tumorigenesis of cervical cancer by downregulating miR-196a and miR-205. Tumour Biol. 2017; 39:1010428317711315. https://doi.org/10.1177/1010428317711315. [Pubmed]
- 21. Analysis of diagnostic and prognostic value of lncRNA MEG3 in cervical cancer. Oncol Lett. 2020; 20:183. https://doi.org/10.3892/ol.2020.12044. [Pubmed]
- 22. Decreased expression of lncRNA GAS5 predicts a poor prognosis in cervical cancer. Int J Clin Exp Pathol. 2014; 7:6776–83. [Pubmed]
- 23. Long noncoding RNA associated with microvascular invasion in hepatocellular carcinoma promotes angiogenesis and serves as a predictor for hepatocellular carcinoma patients’ poor recurrence-free survival after hepatectomy. Hepatology. 2012; 56:2231–41. https://doi.org/10.1002/hep.25895. [Pubmed]
- 24. Long non-coding RNA MVIH indicates a poor prognosis for non-small cell lung cancer and promotes cell proliferation and invasion. Tumour Biol. 2014; 35:7587–94. https://doi.org/10.1007/s13277-014-2009-7. [Pubmed]
- 25. Long non-coding RNA MVIH is associated with poor prognosis and malignant biological behavior in breast cancer. Tumour Biol. 2016; 37:5257–64. https://doi.org/10.1007/s13277-015-4360-8. [Pubmed]
- 26. Long non-coding RNA MVIH acts as a prognostic marker in glioma and its role in cell migration and invasion. Eur Rev Med Pharmacol Sci. 2016; 20:4898–904. [Pubmed]
- 27. Downregulation of long non-protein coding RNA MVIH impairs glioblastoma cell proliferation and invasion through an miR-302a-dependent mechanism. Hum Mol Genet. 2021; 30:46–64. https://doi.org/10.1093/hmg/ddab009. [Pubmed]
- 28. Microvascular invasion in hepatocellular carcinoma overexpression promotes cell proliferation and inhibits cell apoptosis of hepatocellular carcinoma via inhibiting miR-199a expression. Onco Targets Ther. 2015; 8:2303–10. https://doi.org/10.2147/OTT.S86807. [Pubmed]
- 29. Identification of long non-coding RNA MVIH as a prognostic marker and therapeutic target in acute myeloid leukemia. J Clin Lab Anal. 2020; 34:e23113. https://doi.org/10.1002/jcla.23113. [Pubmed]
- 30. Cell kinetics in human breast cancer: comparison between the prognostic value of the cytofluorimetric S-phase fraction and that of the antibodies to Ki-67 and PCNA antigens detected by immunocytochemistry. Int J Cancer. 1994; 57:822–29. https://doi.org/10.1002/ijc.2910570610. [Pubmed]
- 31. Ki67 index and S-phase fraction in human breast carcinomas. Comparison and correlations with prognostic factors. Am J Clin Pathol. 1990; 94:681–86. https://doi.org/10.1093/ajcp/94.6.681. [Pubmed]
- 32. LncRNA MVIH knockdown inhibits the malignancy progression through downregulating miR-505 mediated HMGB1 and CCNE2 in acute myeloid leukemia. Transl Cancer Res. 2019; 8:2526–34. https://doi.org/10.21037/tcr.2019.10.12. [Pubmed]
- 33. Besides Pathology: Long Non-Coding RNA in Cell and Tissue Homeostasis. Noncoding RNA. 2018; 4:3. https://doi.org/10.3390/ncrna4010003. [Pubmed]
- 34. Long noncoding RNAs in development and cancer: potential biomarkers and therapeutic targets. Mol Cell Ther. 2015; 3:5. [Pubmed]
- 35. Long noncoding RNA microvascular invasion in hepatocellular carcinoma is an indicator of poor prognosis and a potential therapeutic target in gastric cancer. J Cancer Res Ther. 2019; 15:126–31. https://doi.org/10.4103/0973-1482.204882. [Pubmed]
- 36. LncRNAs AK058003 and MVIH Overexpression in the Blood Samples of Iranian Breast Cancer Patients. Iran Biomed J. 2021; 25:93–98. https://doi.org/10.29252/ibj.25.2.93. [Pubmed]
