Genes & Cancer
Pathogenesis to management of hepatocellular carcinoma
Ben L. Da1, Kelly I. Suchman2, Lawrence Lau3, Atoosa Rabiee4, Aiwu Ruth He5, Kirti Shetty6, Herbert Yu7, Linda L. Wong8, Richard L. Amdur9, James M. Crawford10, Sharon S. Fox10, Gregory M. Grimaldi11, Priya K. Shah11, Jonathan Weinstein12, David Bernstein1, Sanjaya K. Satapathy1, Nyasha Chambwe13, Xiyan Xiang14 and Lopa Mishra14
1 Department of Internal Medicine, Division of Hepatology, Sandra Atlas Bass Center for Liver Diseases and Transplantation, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell Health, Manhasset, NY 11030, USA
2 Department of Internal Medicine, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell Health, Manhasset, NY 11030, USA
3 Department of Surgery, North Shore University Hospital, Northwell Health, Manhasset, NY 11030, USA
4 Department of Gastroenterology and Hepatology, VA Medical Center, Washington, DC 20422, USA
5 Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC 20007, USA
6 Division of Gastroenterology and Hepatology, University of Maryland, Baltimore, MD 21201, USA
7 Department of Epidemiology, University of Hawaii Cancer Center, Honolulu, HI 96813-5516, USA
8 Department of Surgery, University of Hawaii, Honolulu, HI 96813-5516, USA
9 Quantitative Intelligence, The Institutes for Health Systems Science and Bioelectronic Medicine, The Feinstein Institutes for Medical Research, Northwell Health, Manhasset, NY 10022, USA
10 Department of Pathology and Laboratory Medicine, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, NY 11549, USA
11 Department of Radiology, Northwell Health, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Manhasset, NY 11030, USA
12 Division of Vascular and Interventional Radiology, Department of Radiology, Northwell Health, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Manhasset, NY 11030, USA
13 The Institute of Molecular Medicine, The Feinstein Institutes for Medical Research, Northwell Health, Manhasset, NY 11030, USA
14 The Institute for Bioelectronic Medicine, The Feinstein Institutes for Medical Research and Cold Spring Harbor Laboratory, Department of Medicine, Division of Gastroenterology and Hepatology, Northwell Health, Manhasset, NY 11030, USA
Correspondence to: Lopa Mishra, email: [email protected]
Keywords: pathogenesis; HCC management; genomic heterogeneity; targeted therapy
Received: September 07, 2022
Accepted: November 17, 2022
Published: December 13, 2022
Copyright: © 2022 Da et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ABSTRACT
Hepatocellular carcinoma (HCC) is the most common primary liver cancer whose incidence continues to rise in many parts of the world due to a concomitant rise in many associated risk factors, such as alcohol use and obesity. Although early-stage HCC can be potentially curable through liver resection, liver-directed therapies, or transplantation, patients usually present with intermediate to advanced disease, which continues to be associated with a poor prognosis. This is because HCC is a cancer with significant complexities, including substantial clinical, histopathologic, and genomic heterogeneity. However, the scientific community has made a major effort to better characterize HCC in those aspects via utilizing tissue sampling and histological classification, whole genome sequencing, and developing viable animal models. These efforts ultimately aim to develop clinically relevant biomarkers and discover molecular targets for new therapies. For example, until recently, there was only one approved systemic therapy for advanced or metastatic HCC in the form of sorafenib. Through these efforts, several additional targeted therapies have gained approval in the United States, although much progress remains to be desired. This review will focus on the link between characterizing the pathogenesis of HCC with current and future HCC management.
INTRODUCTION
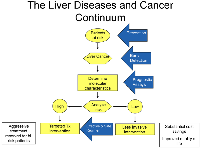
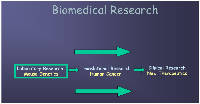
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and the third most common cause of cancer death [1]. The rate of HCC continues to rise, especially in the United States and Central America [2]. Worldwide, the number of new primary liver cancer cases among men and women in 2020 was 577,522 and 252,658, respectively [1]. Most cases are diagnosed in the advanced stages. The presumed reason for the increasing rate of HCC is the rising incidence of liver disease related to alcohol overuse and metabolic syndrome, associated with a dramatic rise in obesity and diabetes mellitus [3–6]. New approaches harnessing large-scale epidemiological studies, histological classification, genomics, biomarkers, and animal models have provided new insights into the development of HCC. These advances are expected to alter the future of HCC management (Figures 1 and 2). This review will focus on the link between the pathogenesis and management of HCC.
Tissue is the issue
The development of HCC typically follows a path from liver inflammation and fibrosis toward a disordered nodular liver architecture (cirrhosis), with the potential progression of nodules through dysplasia to cancer [7]. Dysplastic nodules are further classified into low and high-grade, with high-grade nodules having a much higher risk of progressing to HCC. Once malignant, HCC becomes capable of invasion into adjacent areas of the liver and, more ominously, invasion into the vasculature with the potential for widespread dissemination [8]. There are multiple morphological subtypes of HCC, including biphenotypic (combined HCC and CCA), cirrhotomimetic (cancer cells infiltrating many parts of a lobe or the entire liver), and more. These are depicted in Table 1 [9]. HCC is the major type of primary liver cancer. In contrast, another type, cholangiocarcinoma (CCA), develops from the bile ducts. When present in the liver, CCA is referred to as intrahepatic CCA and represents 5–10% of all liver cancer cases. Distinguishing between CCA and HCC is critically important as the difference determines the course of treatment [10].
Traditionally, the diagnosis of HCC was primarily made with characteristic findings on contrast-enhanced imaging, as tumor biopsies were done infrequently due to a risk of bleeding and needle-track seeding of the tumor [11]. However, tissue sampling is again indicated owing to the need for histological classification and genomic testing to select targeted therapy, guide management, and provide an informed prognosis. The development of coaxial needle techniques has resulted in negligible risks of tumor dissemination [12, 13], leading to greater acceptance of tumor biopsies. Recently, there has been increasing interest in the molecular analysis of tumor-derived circulating tumor cells (CTCs), extracellular vesicles (EVs), and cell-free circulating nucleic acids (cfDNA or cfRNA), termed “liquid biopsy” [14]. Although these approaches might be complementary, particularly when tissue samples are insufficient or unsuitable for biomarker testing [15], tissue-based analysis remains the preferred method for molecular testing when tissue is available [16].
Genetic alterations in HCC
Human HCC has significant clinical, histopathologic, and genomic heterogeneity. Cancer cells can vary from well to poorly differentiated and may arise from multiple cell lines [17]. For example, cancer stem cells (that self-renew and can also generate various progeny) are believed to give rise to nearly 40% of HCCs. They may be one of the factors responsible for tumor heterogeneity and resistance to therapy [18, 19]. In addition, multiple genetic events have been associated with the development and pathogenesis of HCC, including mutations, amplifications, deletions, chromosomal rearrangements, and aberrant methylation [20]. Advancements in our understanding of these events guide the development of therapies based on the presence of individual or groups of genetic alterations [21]. We will now review some of the genetic alterations associated with the pathogenesis of HCC.
The most common mutations in HCC are somatic mutations in the promoter of the gene encoding telomerase reverse transcriptase (TERT), which are present in 54–60% of all HCC cases [22, 23]. Telomeres protect chromosome ends and become shorter with repeated cell division, while telomerase is the enzyme responsible for maintaining the length of the telomere. Telomere shortening is accentuated in chronic liver injury, resulting in apoptosis and the inability of the liver to regenerate fully, and is likely linked to the pathogenesis of HCC [11].
HCC is also associated with a high frequency of mutations in several other genes. A standard mutational profile, present in 31% of HCCs, is inactivating mutations of TP53, encoding the tumor-suppressor p53. Four other common mutations in HCC activate WNT pathway genes (CTNNB1, AXIN 1/2, APC; 20% of cases) [24, 25]. Other mutations alter a chromatin remodeling pathway (ARID1A and ARID2; 7% and 5% of cases, respectively), while others affect a gene encoding a deubiquitinase (BAP1; 5%) [22, 24, 25].
Calderaro et al. analyzed mutations associated with HCC and correlated the genetic mutations present with their clinical presentation [24]. Tumors with CTNNB1 mutations were large, well-differentiated, cholestatic, and lacked markers of inflammation. Meanwhile, tumors with TP53 mutations were poorly differentiated, multinucleated, and exhibited frequent vascular invasion. Patients with TERT promoter mutations tend to be older, predominantly male, and more likely to be HCV positive. In addition, mutations in the TGF-β pathway occur in up to 40% of HCCs. Alterations in the TGF-β pathway can be characterized by elevated HMGA2 and TERT levels, highlighting the concept of how many pathways can interact to drive HCC [23].
Gene amplification and deletions, as well as chromosomal rearrangements, are structural alterations that play an important role in the carcinogenesis of HCC. Amplification of the genes CCND1 (encoding cyclin D1) was present in 15% of the HCCs studied in one report [25]. Common deletions found in HCC include those found in CDKN2A (encoding both the proteins p16 and p14, tumor suppressors) or RB1 (encoding the tumor suppressor retinoblastoma transcriptional corepressor 1) [19]. Furthermore, a chromosomal rearrangement involving chromosome 19 resulting in the formation of chimeric RNAs is associated with fibrolamellar HCC [26].
Epigenetic alterations include changes in methylation, chromatin remodeling, and micro-RNAs. Aberrant methylation of multiple promoters is associated with advanced HCC, chronic viral hepatitis B or C, and liver cirrhosis [20, 27]. Recent reports indicate that changes in 19 miRNAs correlated with disease outcomes, suggesting that this miRNA signature may be used as a disease prognosticator [28].
The potential of animal models
Animal models allow for the integration of the genomic and molecular characterization of human HCC samples in a way that permits for the discovery and testing of biomarkers and novel HCC therapies. Examples of animal models that have been developed which resemble liver cancer in humans include the C-Myc/p53, Smad4/PTEN (CCA), PDGFR, TAK1, compromised TGF-β signaling, and MUP-uPA animal models [29–37]. Other animal models that have been developed include a mouse model of NASH and a human stem cell disease that exhibits spontaneous liver cancer and alcohol-induced enhancement for the development of liver cancer [29].
The NASH-HCC model is MUP-uPA mice fed a high-fat diet (HFD), resulting in steatohepatitis resembling human NASH. Its progression to HCC represents a reliable model of NASH-driven HCC that has been used to evaluate HCC-targeting immunotherapies. Cancer stem cell and alcohol-sensitive models are found in mice with compromised TGF-β signaling (Smad2+/−/Smad3+/− mice) [30, 38]. In both cases, these are immune-competent models that can provide opportunities to investigate the crosstalk between cancer cells, their microenvironment, and the immune system, as well as explore potential contributions of the microbiome. Such information is critical for helping us understand the molecular causes responsible for the progression from liver injury to HCC, identify new therapeutic targets, design effective combination strategies and treatment regimens, and determine prevention strategies.
Surveillance
Surveillance using abdominal ultrasound with or without serum alpha-fetoprotein (AFP) levels every six months is recommended by current guidelines for patients with cirrhosis and has been associated with improved survival [39, 40]. Certain populations without cirrhosis, such as those with hepatitis B, are also recommended to undergo HCC screening (depending on age and race) due to an elevated risk of HCC [41].
However, the effectiveness of HCC surveillance in clinical practice is severely limited by a lack of sensitivity and specificity of both ultrasound (particularly lesions under 1 cm) and AFP tests [42]. Ultrasound is also operator-dependent, and sensitivity can depend on the patient’s body habitus. These screening methods are underutilized as only 16.9% of primary care physicians and 51.7% of subspecialty physicians screen correctly per current liver guideline recommendations [43–47]. Finally, approximately 20% of HCCs arise in those without established cirrhosis creating further screening difficulties [48].
Contrast-enhanced computerized tomography (CT) and magnetic resonance imaging (MRI) scans offer better HCC detection and have traditionally allowed for the ability to make a diagnosis. However, the use of these imaging studies is limited by cost, insurance coverage, use of intravenous contrast, exposure to radiation, and claustrophobia [44, 46]. MRI is superior to CT in detecting HCC (particularly for small tumors) in this setting [49].
Due to the limitations of AFP as an HCC screening biomarker, novel biomarkers such as AFP-L3%, des-gamma-carboxy prothrombin (DCP), osteopontin, glycosylated proteins, and circulating tumor cells have been discovered and studied as individual and combination testing [50]. However, all these tests have significant flaws, such as AFP-L3%, which is hindered by low sensitivity in early disease [51]. Attempts have been made to combine biomarkers with clinical factors, such as the GALAD score incorporating age, gender, AFP, AFP-L3%, and DCP [52]. While this score improved the detection abilities of each biomarker alone, it is prone to false positives [52]. Recently, a promising blood-based panel of methylated DNA and protein markers has demonstrated improved early HCC detection capabilities (71% sensitivity and 90% specificity) compared to the GALAD score or AFP alone [53].
Future research should focus on precision screening for HCC considering patient characteristics and the risk of HCC development. Similarly, preventative approaches that eliminate HCC risk factors such as liver disease drivers (e.g., alcohol, HBV, metabolic syndrome, etc.) can substantially reduce rates of HCC [54]. Dietary and pharmacologic approaches to reducing the risk for HCC are also under consideration. Still, their role in clinical management needs to be further validated and defined [55–59].
Treatment of early and intermediate stage HCC
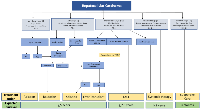
Once diagnosed, treatment of HCC remains primarily based on the Barcelona Clinic Liver Cancer (BCLC) staging (Figure 3) [60, 61]. Individual academic centers often have multidisciplinary tumor boards where care can be discussed and treatment plans formulated. Curative therapy can be achieved for early (usually less than 3 cm) HCC via ablation or surgical resection for eligible patients. When possible, surgery offers the best overall and disease-free survival in the treatment of HCC. Surgical resection is generally limited to patients with a solitary tumor confined to the liver in the setting of well-compensated Child-Pugh Class A cirrhosis without significant portal hypertension and sufficient liver remnant volume after resection. In these selected patients, surgical resection has comparable survival to liver transplantation without the disadvantages of waiting for a suitable donor liver and the need for post-transplant immunosuppression [62, 63].
The key considerations in deciding between surgical resection and liver transplantation include an assessment of tumor stage, location, the severity of liver disease, and availability of donor liver grafts. Liver transplantation completely removes both the tumor and the diseased, tumorigenic liver. Due to the limited supply of donor livers and the need to share donor livers with non-HCC patients, the current Organ Procurement and Transplantation Network (OPTN) policy limits the eligibility of transplant candidates to those with T2 HCC lesions (a solitary lesion measuring 2 to 5 cm, or up to three lesions measuring 1 to 3 cm). This was based on excellent actuarial overall (75%) and recurrence-free survival (83%) observed in patients transplanted with HCC within “Milan Criteria” [64].
The allocation of donor livers is based on the Model for End-stage Liver Disease-sodium (MELD- Na) score. Because HCC patients often have relatively low MELD-Na scores compared to those with end-stage cirrhosis, these patients can be granted “exception points” depending on tumor size/number and after six months of waiting on the list (Table 2) [64]. The criteria for liver transplantation continue to evolve to balance the risk of death in end-stage cirrhosis patients with the risk of tumor progression in HCC patients. Liver-directed therapies (LDT) can be utilized to bridge patients to transplant or to “down-stage” HCC that is outside of Milan criteria but within “UCSF criteria” for liver transplantation (Table 2) [64, 65]. Recently, the important role of biomarkers in liver transplantation has been recognized with high AFP (>1000 ng/mL) used as a surrogate for aggressive tumor biology and disqualifying patients for MELD exception points. As the transplant criteria for HCC evolve, the future likely lies in utilizing a combination of disease prognosticating biomarkers, tumor size and number, and response to LDT [66].
Liver-directed therapies (LDT) can be used for curative intent, bridge to transplant, downstage to transplant, and palliative purposes. Ablation, bland embolization, transarterial chemoembolization (TACE), drug-eluting bead transarterial chemoembolization (DEB-TACE), and Yttrium90 transarterial radioembolization (TARE) are therapies to treat HCC. Ablation is considered the primary treatment for very early-stage HCC (BCLC 0) not amenable for resection or transplant. If ablation is not feasible, transarterial treatments such as TACE, DEB-TACE, and TARE are performed with notably high efficacy at high radiation doses administered during TARE [67]. This treatment approach applies to early-stage HCC (BCLC Stage A) patients and patients who are not surgical candidates. Ablation is less effective for larger masses, and transarterial treatments are usually the preferred treatment for those patients.
TACE has been the standard of care in intermediate-stage HCC (BCLC stage B) based on improvements in overall survival (OS) compared to supportive care alone in two randomized studies conducted before the approval of sorafenib. No high-level evidence shows improved survival of TACE compared to DEB-TACE. However, a recent phase 2 randomized controlled study (TRACE) demonstrated improved median overall survival and superior tumor control with TARE compared to DEB-TACE in BCLC Stage A and B patients who were not surgical or ablation candidates [68].
Predicting surgical outcomes based on molecular alterations
Several studies have examined the correlation between genetic changes within tumor and non-tumor tissue and outcomes following hepatic resection and orthotopic liver transplantation (OLT). Mutations in RB1 and TP53 have been associated with an increased risk of tumor recurrence and decreased survival. Transcriptome signatures from tumor tissue correlate with tumor aggressiveness and early recurrence. Expression levels of 5 specific genes (5-gene score) in tumor tissue have been shown to correlate with early tumor recurrence and overall survival in patients undergoing hepatic resection [69]. An HCC subtype characterized by TP53 mutation, high fractional allelic loss, significant global hypomethylation, and absence of CTNNB1 mutation were noted to predict shorter recurrence-free survival in patients who underwent liver transplantation [70]. Whole transcriptomic profiling of gene expression signatures has identified progenitor cell markers as predictors of recurrence after OLT [71]. However, these studies are based on pathological analysis of the explanted liver and, therefore, cannot be utilized pre-transplant in decision-making regarding transplant eligibility.
Immunotherapy and systemic targeted treatments for advanced-stage HCC
The immune component of the HCC microenvironment has been of great interest with the recent approval of immunotherapies, such as immune checkpoint inhibitors, for advanced stage HCC (BCLC Stage C) (Figure 4). However, only a subset of HCCs have high levels of immune cell infiltration, and the response rate of HCCs to immunotherapies is only around 10–35% [72, 73]. Thus, a key to the safe and effective use of such treatments will be correctly identifying patients most likely to exhibit a positive response.
Sorafenib is a multi-targeted tyrosine kinase inhibitor (TKI) that was the first approved target therapy and the standard of care for advanced HCC (Figure 4). Sorafenib exerts antiproliferative (RAF1, MEK, ERK), anti-angiogenic (VEGFR2 and PDGFR), and pro-apoptotic effects [74]. The SHARP trial was a landmark study that led to its approval and demonstrated a modest survival benefit of sorafenib versus placebo (median OS 10.7 vs. 7.9 months, P < 0.001) [75]. Sorafenib is indicated as a first-line treatment option for patients with well-preserved liver function (Child-Pugh A) and advanced tumors (BCLC C) or tumors at an intermediate stage (BCLC B) that failed LDT.
The approval of Sorafenib in 2007 was followed by many largely unsuccessful late-stage trials assessing novel targeted therapies. However, starting in 2017, six additional systemic agents were approved for advanced-stage HCC, including for first-line therapy (lenvantinib) and second-line therapy (cabozantinib, regorafenib, and ramucirumab if AFP >400 ng/mL), as well as two immune checkpoint inhibitors (ICI) for second-line therapy (nivolumab and pembrolizumab) in the accelerated approval setting [76, 77]. However, this was followed by two recent phase 3 trials investigating the two approved anti-programmed death-1 (anti-PD-1) monoclonal antibody immunotherapies, nivolumab (1st line indication), and pembrolizumab (2nd line indication), that failed to reach their primary endpoints despite promising phase 2 results [78, 79].
In 2020, a breakthrough occurred in the landmark IMbrave150 study of atezolizumab (anti-PD-L1) + bevacizumab (anti-VEGF) versus sorafenib in advanced HCC [80]. This study demonstrated improved OS at 12 months of 67.2% vs. 54.6% with atezolizumab/bevacizumab and better progression-free survival (PFS) of 6.8 months vs. 4.3 months. This study required assessment and treatment of esophageal or gastric varices before enrollment due to the bevacizumab component. This study only included patients with preserved liver function with Child-Pugh A cirrhosis. A network meta-analysis of targeted immunotherapies also found superior OS with the combination of atezolizumab + bevacizumab used in the first-line setting compared to sorafenib, lenvatinib, and nivolumab [81]. The atezolizumab + bevacizumab combination is the first-line standard of care in patients with unresectable or metastatic HCC. Recently presented phase 3 data (HIMALAYA trial) with the novel immunotherapeutic combination of tremelimumab plus durvalumab demonstrated better OS and PFS compared to sorafenib [82]. The FDA approved this combination on Oct 21, 2022, making it an attractive first-line option in advanced HCC.
Current Treatment Landscape: The choice of therapy is dictated by hepatic synthetic function, portal hypertension, performance status and tumor burden. ASCO guidelines recommend atezolizumab-bevacizumab or TKIs (sorafenib/lenvatinib) as first line therapy. The choice of second-line therapy is dependent on initial treatment. Those treated with durvalumab-tremelimumab or atezolizumab-bevacizumab may be offered TKIs such as sorafenib, lenvatinib, regorafenib or cabozantinib. If the patient progresses despite sorafenib or lenvatinib as first line therapy and has contraindications to immune checkpoint inhibitors, regorafenib or cabozantinib could be an option, with ramucirumab utilized in those with AFP level more than 400 ng/mL. For those patients who progress on TKIs, dual immunotherapy (nivolumab-iplimumab ) is preferred as it has been shown to produce higher ORR. If unable to tolerate this combination, pembrolizumab monotherapy may be utilized as an alternative [83].
Despite these advances, there remain many other potential targets for HCC therapy. For example, the aberrant activity of the TGF-β pathway is involved in nearly 40% of HCCs, and deficiency of this pathway is observed in the cancer stem cell signature of HCC. Thus, this pathway represents a potential drug target. The JAK/STAT pathway is a signaling pathway that is aberrantly activated in some HCCs and represents a potential intervention target [84, 85]. Tumor sensitivity may differ between patients, so we can tailor radiation dose by biomarkers in the future (e.g., ADC, diffusion coefficient). The Cancer Genome Atlas (TCGA) genomics could provide vital clues in identifying specific groups that can be targeted with modalities such as radiation. For instance, TCGA analyses of 9,125 cancers, including HCCs, revealed that inactivation of the TGF-β pathway correlates with aberrant DNA crosslinking repair members and sirtuins and an increase in ‘Stemness’ in potentially 40% of HCCs. These HCCs may be more responsive to radiation therapy.
Precision oncology in HCC
While personalized medicine has become a practical reality in other cancers, HCC has been difficult to target with specific treatments based on molecular profiling. One of the main reasons for targeted therapies’ failure is tumor heterogeneity (inter and intra-subject). A challenge to developing therapies is that somatic mutations occur in genes whose products are not easily or safely druggable, such as mutated forms of TERT, TP53, CTNNB1, and MYC. New strategies to target driver genes and pathways, such as micro-RNA-based therapeutics, are in development for other cancers and may apply to HCC in the future.
Neoantigens that arise from mutations in HCC tumors have emerged as important targets for future combinatorial immunotherapy [86–89]. However, most tumor mutations are not shared across individuals. Because of HLA diversity (i.e., the small proportion of shared neoantigens are recognized only by T cells of specific subsets of patients), immunogenic tumor neoantigens generally must be selected based on each patient’s tumor [90, 91]. Vaccines must also be customized. Nevertheless, several clinical trials conducted with patients with solid tumors and melanoma have demonstrated that personal neoantigen-directed vaccines are feasible, safe, and immunogenic [92–94]. Phase II trials are currently in progress for HCC harnessing this technology [87, 95].
Similar clinical trials are in progress using oncolytic viruses in combination with chemotherapy and immunotherapy [96, 97]. Recombinant oncolytic viruses harness the disparity of genetic mutations and molecular activity between liver cancer cells and normal cells, displaying a reasonable specificity for targeting HCCs. Oncolytic vaccinia virus such as JX-594 lacks the TK gene and expresses granulocyte-macrophage colony-stimulating factor (GM-CSF), specifically targets liver cancer cells with high cellular TK activity and activated EGFR signaling, which facilitates its replication [98].
Novel targets for molecular therapies
In this section, we will briefly highlight promising, well-tolerated active compounds which target driver genes and pathways in HCC and are currently being studied for clinical application (Table 3).
MET inhibitors
The tyrosine kinase receptor c-MET (MET) through its ligand, hepatocyte growth factor (HGF) leads to HCC progression by promoting cellular proliferation and invasion, as well as by mediating tyrosine kinase resistance. The clinical utility of older MET inhibitors had been limited by their toxicity. However, newer and more selective oral compounds have demonstrated promising results with good tolerability. Recent phase 1b/2 trials of tepotinib (FDA-approved for non- small cell lung cancer) in treatment naïve Asian patients (https://clinicaltrials.gov/ NCT01988493) [99] and in sorafenib resistant non-Asian cohorts with HCC (https://clinicaltrials.gov/: NCT02115373) [100] showed that 63.3% of patients were progression-free at 12 weeks. However, a low overall response rate (ORR of 8%) and disease control rate (DCR of 27%) suggest that selective MET inhibition is effective in only a minority of even those patients selected on the basis of MET positivity on immunochemistry [100].
Fibroblast growth factor (FGF) receptor inhibitors
The FGF family and its receptors (FGFR) are involved in multiple carcinogenic pathways. Aberrant FGF/FGFR signaling has been noted to enhance HCC cell invasion by suppressing E-cadherin expression and promoting the expression of epithelial-to-mesenchymal transition-related genes. BLU-554 (also known as fisogatinib) is the most advanced of the clinically translatable FGFR4 inhibitors and is still being investigated in a phase I study for the treatment of advanced HCC (https://clinicaltrials.gov/ Identifier: NCT02508467). Another compound being studied in a phase 1 study for advanced HCC is H3B-6527 (https://clinicaltrials.gov/ Identifier: NCT02834780). FGF401 (also known as roblitinib) is a reversible covalent inhibitor, displaying more than a 100-fold selectivity for FGFR4 compared to FGFR1-3. FGF401 exhibits potent antitumor activity in HCC with aberrant FGF19 overexpression. Novartis pharmaceuticals sponsored a clinical trial of FGF401 from 2014 to 2020. This study aimed to test the maximum tolerated dose and/or recommended phase II dose and efficacy of FGF401 as a monotherapy or in combination with PDR001 in HCC patients positive for FGFR4 and KLB (https://clinicaltrials.gov/ Identifier: NCT02325739). This study has been completed but conclusive data have not been published, and the phase II part of the FGF401 + PDR001 combination was halted due to commercial reasons.
Galunisertib (LY2157299 Monohydrate), a TGF‐β receptor 1 inhibitor which had shown promise in sorafenib resistant HCC, has also been withdrawn from clinical use. From these therapeutic trials, we may conclude that molecular therapy for HCC has potential, but is fraught with challenges.
Disparities in HCC incidence and mortality are prevalent
Significant disparities in HCC incidence and mortality due to geographic location, sex, race, ethnicity, sociocultural contexts, and clinical factors exist. Globally, liver cancer incidence and mortality rates are double to triple in men compared to women [1]. High-risk areas for HCC include China, the Republic of Korea, and sub-Saharan Africa [1]. Within the U.S., surveillance data indicate widespread racial and ethnicity-based liver and intrahepatic bile duct cancer disparities [101]. Liver cancer incidence and mortality are highest in the American Indian or Alaska Native (AI/AN) population, with an almost two-fold higher rate than Whites in the most recent data surveys (2014–2019) [102]. Similarly, liver cancer incidence and mortality are also very high for the Hispanic and Asian/Pacific Islanders [102]. Additionally, US-born Hispanic individuals have been reported to have higher HCC incidence compared to foreign-born individuals [103]. These differences can partially be explained by the geographical variation in the prevalence of the known HCC risk factors. Countries with high HBV or HCV prevalence also had high HCC incidence [104]. The high prevalence of HCC in Asian individuals in the U.S. can also be explained by a high number of foreign-born individuals from countries with high HBV prevalence [105, 106]. In the US, there is evidence of a higher rate of metabolic disorders related to HCC etiology in Hispanics and higher rates of chronic infection with HBV or HCV in Hispanic and African American men [107, 108].
Variability in clinical factors, including access to and quality of healthcare, also account for disparities in HCC incidence and mortality. Hispanic and Black HCC patients are less likely to be diagnosed early or undergo curative treatment than white patients [109]. Black or Hispanics with early-stage HCC living in counties with poor social determinants of health (SDOH) are less likely to receive surgical intervention than in other counties [110]. A similar dichotomy exists between rural and suburban dwellers, with rural liver cancer patients less likely to undergo treatment resulting in higher rural mortality due to liver cancer compared to patients in more urban and peri-urban areas [111].
While differential incidence and mortality of HCC in different populations are likely caused by differential exposure to infectious agents, the prevalence of metabolic syndrome and chronic liver disease, and disparities in health care quality, the role of genetic and other molecular mechanisms cannot be ruled out. For example, the mutational frequency of TP53 in HCC in the TCGA cohort is significantly different between different racial and ethnic groups (Black or African Americans, 70.6%, Asians, 36.5%, and Caucasians, 22.8%) [112], suggesting differential tumor drivers according to the population of origin. Further studies examining the genetic and biological basis of race and ethnicity-based HCC disparities are needed to determine tumor biological factors that account for the unexplained causes of HCC disparities at the molecular level, such that interventional strategies can be designed appropriately. Awareness of these disparities and their causes should influence HCC patient management by focusing on interventions that can contribute to addressing inequities in cancer prevention, screening, and treatment in HCC.
FUTURE DIRECTIONS
While surgical therapies can be curative, only a small proportion of patients with HCC will qualify. Despite early detection and chemoprevention efforts, HCC incidence continues to arise, frequently in more advanced stages. The prognosis of intermediate to advanced HCC remains poor, and there is much room for improvement. Research through histological classification, whole genome characterization, and the development of multiple viable animal models has led to substantial progress on the pathogenesis of HCC over the last decade. This has culminated in the approval of multiple new treatments for HCC. However, there are several significant challenges to future progress in this complex malignancy. The limited tissue specimens for measuring biomarkers discovered through publicly-available databases for HCC remains a major issue. The absence of clinically validated commercially available biomarkers with high sensitivity and specificity is an important reason why HCC diagnosis and treatments have lagged decades behind other solid cancers. There remains an urgent need for integrating biomarkers found in HCC into clinical practice. In addition, diversifying the study and clinical trial cohorts for adequate representation of racial and ethnic groups disproportionately affected by HCC is critical to ensure that biomarkers developed and personalized medicine approaches will be pertinent in these populations. Successfully harnessing integrated approaches that combine bioinformatics analysis with in vivo animal models and in vitro biological and biochemical methods is the key to effectively developing future cures for this lethal cancer.
Abbreviations
HCC: hepatocellular carcinoma; IACS: interaction analysis; GEM: genetic and epigenetic mechanisms; US: ultrasound; CT: computed tomography; MRI: magnetic resonance imaging; HCV: hepatitis C virus; HBV: hepatitis B virus; ETOH: ethanol alcohol.
Author contributions
LM and BD contributed to the topic formulation. BD, KS, LL, JW, and LM contributed to the review’s design, sampling relevant studies, information collection, and drafting the article. AR, ARH, KS, HY, LLW, RLA, JMC, SSF, GMG, PKS, DB, SKS, NC, XX, and LM revised and edited the article critically for presentation, interpretation, discussion, and implication for future research. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
CONFLICTS OF INTEREST
Authors have no conflicts of interest to declare.
FUNDING
This work was supported by NIH grants R01AA023146 (L. Mishra), R01CA236591 (L. Mishra), and U01CA230690 (L. Mishra).


- 1. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021; 71:209–49. https://doi.org/10.3322/caac.21660. [Pubmed]
- 2. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011; 54:463–71. https://doi.org/10.1002/hep.24397. [Pubmed]
- 3. Qidong hepatitis B virus infection cohort: a 25-year prospective study in high risk area of primary liver cancer. Hepatoma Res. 2018; 4:4. https://doi.org/10.20517/2394-5079.2017.50. [Pubmed]
- 4. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019; 156:477–91.e1. https://doi.org/10.1053/j.gastro.2018.08.065. [Pubmed]
- 5. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2016; 14:124–31.e1. https://doi.org/10.1016/j.cgh.2015.07.019. [Pubmed]
- 6. A Structured Literature Review of the Epidemiology and Disease Burden of Non-Alcoholic Steatohepatitis (NASH). Adv Ther. 2019; 36:1574–94. https://doi.org/10.1007/s12325-019-00960-3. [Pubmed]
- 7. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021; 7:6. https://doi.org/10.1038/s41572-020-00240-3. [Pubmed]
- 8. Intrahepatic Cholangiocarcinoma: Continuing Challenges and Translational Advances. Hepatology. 2019; 69:1803–15. https://doi.org/10.1002/hep.30289. [Pubmed]
- 9. Morphologic Subtypes of Hepatocellular Carcinoma. Gastroenterol Clin North Am. 2017; 46:365–91. https://doi.org/10.1016/j.gtc.2017.01.009. [Pubmed]
- 10. Characterisation of dysplastic liver nodules using low-pass DNA sequencing and detection of chromosome arm-level abnormalities in blood-derived cell-free DNA. J Pathol. 2021; 255:30–40. https://doi.org/10.1002/path.5734. [Pubmed]
- 11. Imaging and Management of Liver Cancer. Semin Ultrasound CT MR. 2020; 41:122–38. https://doi.org/10.1053/j.sult.2019.12.002. [Pubmed]
- 12. Lack of tumor seeding of hepatocellular carcinoma after percutaneous needle biopsy using coaxial cutting needle technique. AJR Am J Roentgenol. 2006; 187:1184–87. https://doi.org/10.2214/AJR.05.1347. [Pubmed]
- 13. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut. 2008; 57:1592–96. https://doi.org/10.1136/gut.2008.149062. [Pubmed]
- 14. Emerging liquid biopsy techniques for early detection of hepatocellular carcinoma, prognostication, and disease monitoring. Clin Liver Dis (Hoboken). 2022; 20:18–20. https://doi.org/10.1002/cld.1232. [Pubmed]
- 15. Expert opinion on NSCLC small specimen biomarker testing - Part 2: Analysis, reporting, and quality assessment. Virchows Arch. 2022; 481:351–66. https://doi.org/10.1007/s00428-022-03344-1. [Pubmed]
- 16. Comparison of solid tissue sequencing and liquid biopsy accuracy in identification of clinically relevant gene mutations and rearrangements in lung adenocarcinomas. Mod Pathol. 2021; 34:2168–74. https://doi.org/10.1038/s41379-021-00880-0. [Pubmed]
- 17. Liver stem cells: implications for hepatocarcinogenesis. Stem Cell Rev. 2005; 1:253–60. https://doi.org/10.1385/SCR:1:3:253. [Pubmed]
- 18. Machine Learning Identifies Stemness Features Associated with Oncogenic Dedifferentiation. Cell. 2018; 173:338–54.e15. https://doi.org/10.1016/j.cell.2018.03.034. [Pubmed]
- 19. Analysis of Genomes and Transcriptomes of Hepatocellular Carcinomas Identifies Mutations and Gene Expression Changes in the Transforming Growth Factor-β Pathway. Gastroenterology. 2018; 154:195–210. https://doi.org/10.1053/j.gastro.2017.09.007. [Pubmed]
- 20. Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell. 2017; 169:1327–41.e23. https://doi.org/10.1016/j.cell.2017.05.046. [Pubmed]
- 21. Targeted therapies for hepatocellular carcinoma. Gastroenterology. 2011; 140:1410–26. https://doi.org/10.1053/j.gastro.2011.03.006. [Pubmed]
- 22. Mutations in TP53, CTNNB1 and PIK3CA genes in hepatocellular carcinoma associated with hepatitis B and hepatitis C virus infections. Genomics. 2013; 102:74–83. https://doi.org/10.1016/j.ygeno.2013.04.001. [Pubmed]
- 23. A Pan-Cancer Analysis Reveals High-Frequency Genetic Alterations in Mediators of Signaling by the TGF-β Superfamily. Cell Syst. 2018; 7:422–37.e7. https://doi.org/10.1016/j.cels.2018.08.010. [Pubmed]
- 24. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J Hepatol. 2017; 67:727–38. https://doi.org/10.1016/j.jhep.2017.05.014. [Pubmed]
- 25. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012; 44:694–98. https://doi.org/10.1038/ng.2256. [Pubmed]
- 26. Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science. 2014; 343:1010–14. https://doi.org/10.1126/science.1249484. [Pubmed]
- 27. DNA methylation biomarkers for hepatocellular carcinoma. Cancer Cell Int. 2018; 18:140. https://doi.org/10.1186/s12935-018-0629-5. [Pubmed]
- 28. Association of MicroRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis, and patient survival. Clin Cancer Res. 2008; 14:419–27. https://doi.org/10.1158/1078-0432.CCR-07-0523. [Pubmed]
- 29. Preclinical models of non-alcoholic fatty liver disease. J Hepatol. 2018; 68:230–37. https://doi.org/10.1016/j.jhep.2017.10.031. [Pubmed]
- 30. TGF-β/β2-spectrin/CTCF-regulated tumor suppression in human stem cell disorder Beckwith-Wiedemann syndrome. J Clin Invest. 2016; 126:527–42. https://doi.org/10.1172/JCI80937. [Pubmed]
- 31. Reciprocal regulation by TLR4 and TGF-β in tumor-initiating stem-like cells. J Clin Invest. 2013; 123:2832–49. https://doi.org/10.1172/JCI65859. [Pubmed]
- 32. Vitamin D Deficiency Promotes Liver Tumor Growth in Transforming Growth Factor-β/Smad3-Deficient Mice Through Wnt and Toll-like Receptor 7 Pathway Modulation. Sci Rep. 2016; 6:30217. https://doi.org/10.1038/srep30217. [Pubmed]
- 33. Characterization of HCC Mouse Models: Towards an Etiology-Oriented Subtyping Approach. Mol Cancer Res. 2019; 17:1493–502. https://doi.org/10.1158/1541-7786.MCR-18-1045. [Pubmed]
- 34. Mechanisms of MAFG Dysregulation in Cholestatic Liver Injury and Development of Liver Cancer. Gastroenterology. 2018; 155:557–71.e14. https://doi.org/10.1053/j.gastro.2018.04.032. [Pubmed]
- 35. IL6-mediated inflammatory loop reprograms normal to epithelial-mesenchymal transition+ metastatic cancer stem cells in preneoplastic liver of transforming growth factor beta-deficient β2-spectrin+/- mice. Hepatology. 2017; 65:1222–36. https://doi.org/10.1002/hep.28951. [Pubmed]
- 36. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell. 2014; 26:331–43. https://doi.org/10.1016/j.ccr.2014.07.001. [Pubmed]
- 37. Targeting β-catenin in hepatocellular cancers induced by coexpression of mutant β-catenin and K-Ras in mice. Hepatology. 2017; 65:1581–99. https://doi.org/10.1002/hep.28975. [Pubmed]
- 38. Loss of the transforming growth factor-β effector β2-Spectrin promotes genomic instability. Hepatology. 2017; 65:678–93. https://doi.org/10.1002/hep.28927. [Pubmed]
- 39. Improved Survival in Patients with Viral Hepatitis-Induced Hepatocellular Carcinoma Undergoing Recommended Abdominal Ultrasound Surveillance in Ontario: A Population-Based Retrospective Cohort Study. PLoS One. 2015; 10:e0138907. https://doi.org/10.1371/journal.pone.0138907. [Pubmed]
- 40. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018; 68:723–50. https://doi.org/10.1002/hep.29913. [Pubmed]
- 41. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016; 63:261–83. https://doi.org/10.1002/hep.28156. [Pubmed]
- 42. Pitfalls in surveillance for hepatocellular carcinoma: How successful is it in the real world? Clin Mol Hepatol. 2017; 23:239–48. https://doi.org/10.3350/cmh.2017.0008. [Pubmed]
- 43. Cost-Effectiveness of Risk Score-Stratified Hepatocellular Carcinoma Screening in Patients with Cirrhosis. Clin Transl Gastroenterol. 2017; 8:e101. https://doi.org/10.1038/ctg.2017.26. [Pubmed]
- 44. MRI With Liver-Specific Contrast for Surveillance of Patients With Cirrhosis at High Risk of Hepatocellular Carcinoma. JAMA Oncol. 2017; 3:456–63. https://doi.org/10.1001/jamaoncol.2016.3147. [Pubmed]
- 45. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012; 27:861–67. https://doi.org/10.1007/s11606-011-1952-x. [Pubmed]
- 46. Improved Detection of Hepatocellular Carcinoma by Using a Longitudinal Alpha-Fetoprotein Screening Algorithm. Clin Gastroenterol Hepatol. 2016; 14:469–75.e2. https://doi.org/10.1016/j.cgh.2015.07.049. [Pubmed]
- 47. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology. 2018; 154:1706–18.e1. https://doi.org/10.1053/j.gastro.2018.01.064. [Pubmed]
- 48. Hepatocellular carcinoma in non-cirrhotic liver: A comprehensive review. World J Hepatol. 2019; 11:1–18. https://doi.org/10.4254/wjh.v11.i1.1. [Pubmed]
- 49. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology. 2015; 275:97–109. https://doi.org/10.1148/radiol.14140690. [Pubmed]
- 50. Evaluation of the Combined Application of AFP, AFP-L3%, and DCP for Hepatocellular Carcinoma Diagnosis: A Meta-analysis. Biomed Res Int. 2020; 2020:5087643. https://doi.org/10.1155/2020/5087643. [Pubmed]
- 51. Alpha-fetoprotein-L3: Useful or Useless for Hepatocellular Carcinoma? Liver Cancer. 2013; 2:151–52. https://doi.org/10.1159/000343847. [Pubmed]
- 52. GALAD Score for Hepatocellular Carcinoma Detection in Comparison with Liver Ultrasound and Proposal of GALADUS Score. Cancer Epidemiol Biomarkers Prev. 2019; 28:531–38. https://doi.org/10.1158/1055-9965.EPI-18-0281. [Pubmed]
- 53. A Novel Blood-Based Panel of Methylated DNA and Protein Markers for Detection of Early-Stage Hepatocellular Carcinoma. Clin Gastroenterol Hepatol. 2021; 19:2597–605.e4. https://doi.org/10.1016/j.cgh.2020.08.065. [Pubmed]
- 54. Prevention of hepatocellular carcinoma and monitoring of high-risk patients. Hepatol Forum. 2022; 3:33–38. https://doi.org/10.14744/hf.2021.2021.0033. [Pubmed]
- 55. Coffee, including caffeinated and decaffeinated coffee, and the risk of hepatocellular carcinoma: a systematic review and dose-response meta-analysis. BMJ Open. 2017; 7:e013739. https://doi.org/10.1136/bmjopen-2016-013739. [Pubmed]
- 56. Association of Aspirin with Hepatocellular Carcinoma and Liver-Related Mortality. N Engl J Med. 2020; 382:1018–28. https://doi.org/10.1056/NEJMoa1912035. [Pubmed]
- 57. Are isothiocyanates and polyphenols from Brassicaceae vegetables emerging as preventive/therapeutic strategies for NAFLD? The landscape of recent preclinical findings. Food Funct. 2022; 13:8348–62. https://doi.org/10.1039/d2fo01488b. [Pubmed]
- 58. The Pathogenesis of HCC Driven by NASH and the Preventive and Therapeutic Effects of Natural Products. Front Pharmacol. 2022; 13:944088. https://doi.org/10.3389/fphar.2022.944088. [Pubmed]
- 59. Hepatocellular carcinoma chemoprevention by targeting the angiotensin-converting enzyme and EGFR transactivation. JCI Insight. 2022; 7:e159254. https://doi.org/10.1172/jci.insight.159254. [Pubmed]
- 60. Hepatocellular Carcinoma: Molecular Pathogenesis and Therapeutic Advances. Cancers (Basel). 2022; 14:621. https://doi.org/10.3390/cancers14030621. [Pubmed]
- 61. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022; 76:681–93. https://doi.org/10.1016/j.jhep.2021.11.018. [Pubmed]
- 62. Liver transplantation versus liver resection for hepatocellular carcinoma in intention to treat: An attempt to perform an ideal meta-analysis. Liver Transpl. 2017; 23:836–44. https://doi.org/10.1002/lt.24758. [Pubmed]
- 63. Liver Transplantation and Liver Resection for Cirrhotic Patients with Hepatocellular Carcinoma: Comparison of Long-Term Survivals. J Gastrointest Surg. 2018; 22:840–48. https://doi.org/10.1007/s11605-018-3690-4. [Pubmed]
- 64. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996; 334:693–99. https://doi.org/10.1056/NEJM199603143341104. [Pubmed]
- 65. Current approach to down-staging of hepatocellular carcinoma prior to liver transplantation. Curr Opin Organ Transplant. 2008; 13:234–40. https://doi.org/10.1097/MOT.0b013e3282fc2633. [Pubmed]
- 66. What Are the Optimal Liver Transplantation Criteria for Hepatocellular Carcinoma? Clin Liver Dis (Hoboken). 2019; 13:20–25. https://doi.org/10.1002/cld.793. [Pubmed]
- 67. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology. 2021; 74:2342–52. https://doi.org/10.1002/hep.31819. [Pubmed]
- 68. 90Y Radioembolization versus Drug-eluting Bead Chemoembolization for Unresectable Hepatocellular Carcinoma: Results from the TRACE Phase II Randomized Controlled Trial. Radiology. 2022; 303:699–710. https://doi.org/10.1148/radiol.211806. [Pubmed]
- 69. A hepatocellular carcinoma 5-gene score associated with survival of patients after liver resection. Gastroenterology. 2013; 145:176–87. https://doi.org/10.1053/j.gastro.2013.03.051. [Pubmed]
- 70. Molecular Scoring of Hepatocellular Carcinoma for Predicting Metastatic Recurrence and Requirements of Systemic Chemotherapy. Cancers (Basel). 2018; 10:367. https://doi.org/10.3390/cancers10100367. [Pubmed]
- 71. Progenitor cell markers predict outcome of patients with hepatocellular carcinoma beyond Milan criteria undergoing liver transplantation. J Hepatol. 2015; 63:1368–77. https://doi.org/10.1016/j.jhep.2015.07.025. [Pubmed]
- 72. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021; 18:525–43. https://doi.org/10.1038/s41575-021-00438-0. [Pubmed]
- 73. Immunotherapy for hepatocellular carcinoma: recent advances and future perspectives. Ther Adv Med Oncol. 2019; 11:1758835919862692. https://doi.org/10.1177/1758835919862692. [Pubmed]
- 74. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006; 66:11851–58. https://doi.org/10.1158/0008-5472.CAN-06-1377. [Pubmed]
- 75. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–90. https://doi.org/10.1056/NEJMoa0708857. [Pubmed]
- 76. Immunotherapy for advanced hepatocellular carcinoma: a focus on special subgroups. Gut. 2021; 70:204–14. https://doi.org/10.1136/gutjnl-2020-321702. [Pubmed]
- 77. Targeted therapy for hepatocellular carcinoma. Signal Transduct Target Ther. 2020; 5:146. https://doi.org/10.1038/s41392-020-00264-x. [Pubmed]
- 78. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022; 23:77–90. https://doi.org/10.1016/S1470-2045(21)00604-5. [Pubmed]
- 79. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020; 38:193–202. https://doi.org/10.1200/JCO.19.01307. [Pubmed]
- 80. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020; 382:1894–905. https://doi.org/10.1056/NEJMoa1915745. [Pubmed]
- 81. Systemic Therapy and Sequencing Options in Advanced Hepatocellular Carcinoma: A Systematic Review and Network Meta-analysis. JAMA Oncol. 2020; 6:e204930. https://doi.org/10.1001/jamaoncol.2020.4930. [Pubmed]
- 82. Durvalumab plus tremelimumab in unresectable hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2022; 11:592–96. https://doi.org/10.21037/hbsn-22-143. [Pubmed]
- 83. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J Clin Oncol. 2020; 38:4317–45. https://doi.org/10.1200/JCO.20.02672. [Pubmed]
- 84. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006; 130:1117–28. https://doi.org/10.1053/j.gastro.2006.01.006. [Pubmed]
- 85. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007; 27:55–76. https://doi.org/10.1055/s-2006-960171. [Pubmed]
- 86. Neoantigens as potential vaccines in hepatocellular carcinoma. J Immunother Cancer. 2022; 10:e003978. https://doi.org/10.1136/jitc-2021-003978. [Pubmed]
- 87. Combination Neoantigen-Based Dendritic Cell Vaccination and Adoptive T-Cell Transfer Induces Antitumor Responses Against Recurrence of Hepatocellular Carcinoma. Cancer Immunol Res. 2022; 10:728–44. https://doi.org/10.1158/2326-6066.CIR-21-0931. [Pubmed]
- 88. High-affinity neoantigens correlate with better prognosis and trigger potent antihepatocellular carcinoma (HCC) activity by activating CD39+CD8+ T cells. Gut. 2021; 70:1965–77. https://doi.org/10.1136/gutjnl-2020-322196. [Pubmed]
- 89. Unique TP53 neoantigen and the immune microenvironment in long-term survivors of Hepatocellular carcinoma. Cancer Immunol Immunother. 2021; 70:667–77. https://doi.org/10.1007/s00262-020-02711-8. [Pubmed]
- 90. Neoantigen vaccine: An emerging immunotherapy for hepatocellular carcinoma. World J Gastrointest Oncol. 2021; 13:673–83. https://doi.org/10.4251/wjgo.v13.i7.673. [Pubmed]
- 91. Targeting Neoantigens in Hepatocellular Carcinoma for Immunotherapy: A Futile Strategy? Hepatology. 2021; 73:414–21. https://doi.org/10.1002/hep.31279. [Pubmed]
- 92. A Phase Ib Trial of Personalized Neoantigen Therapy Plus Anti-PD-1 in Patients with Advanced Melanoma, Non-small Cell Lung Cancer, or Bladder Cancer. Cell. 2020; 183:347–62.e24. https://doi.org/10.1016/j.cell.2020.08.053. [Pubmed]
- 93. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017; 547:217–21. https://doi.org/10.1038/nature22991. [Pubmed]
- 94. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019; 565:234–39. https://doi.org/10.1038/s41586-018-0792-9. [Pubmed]
- 95. Personalized neoantigen vaccine prevents postoperative recurrence in hepatocellular carcinoma patients with vascular invasion. Mol Cancer. 2021; 20:164. https://doi.org/10.1186/s12943-021-01467-8. [Pubmed]
- 96. Combination Immunotherapy Using Oncolytic Virus for the Treatment of Advanced Solid Tumors. Int J Mol Sci. 2020; 21:7743. https://doi.org/10.3390/ijms21207743. [Pubmed]
- 97. Adjuvant oncolytic virotherapy for personalized anti-cancer vaccination. Nat Commun. 2021; 12:2626. https://doi.org/10.1038/s41467-021-22929-z. [Pubmed]
- 98. The emerging therapeutic potential of the oncolytic immunotherapeutic Pexa-Vec (JX-594). Oncolytic Virother. 2015; 4:25–31. https://doi.org/10.2147/OV.S59640. [Pubmed]
- 99. Randomised Phase 1b/2 trial of tepotinib vs sorafenib in Asian patients with advanced hepatocellular carcinoma with MET overexpression. Br J Cancer. 2021; 125:200–8. https://doi.org/10.1038/s41416-021-01380-3. [Pubmed]
- 100. Phase 1b/2 trial of tepotinib in sorafenib pretreated advanced hepatocellular carcinoma with MET overexpression. Br J Cancer. 2021; 125:190–99. https://doi.org/10.1038/s41416-021-01334-9. [Pubmed]
- 101. Etiology and Outcomes of Hepatocellular Carcinoma in an Ethnically Diverse Population: The Multiethnic Cohort. Cancers (Basel). 2021; 13:3476. https://doi.org/10.3390/cancers13143476. [Pubmed]
- 102. Cancer statistics, 2022. CA Cancer J Clin. 2022; 72:7–33. https://doi.org/10.3322/caac.21708. [Pubmed]
- 103. Disparity in liver cancer incidence and chronic liver disease mortality by nativity in Hispanics: The Multiethnic Cohort. Cancer. 2016; 122:1444–52. https://doi.org/10.1002/cncr.29922. [Pubmed]
- 104. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020; 8:e180–90. https://doi.org/10.1016/S2214-109X(19)30488-7. [Pubmed]
- 105. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology. 2012; 56:422–33. https://doi.org/10.1002/hep.24804. [Pubmed]
- 106. An Updated Assessment of Chronic Hepatitis B Prevalence Among Foreign-Born Persons Living in the United States. Hepatology. 2021; 74:607–26. https://doi.org/10.1002/hep.31782. [Pubmed]
- 107. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer. 2016; 122:1757–65. https://doi.org/10.1002/cncr.29971. [Pubmed]
- 108. The association between etiology of hepatocellular carcinoma and race-ethnicity in Florida. Liver Int. 2020; 40:1201–10. https://doi.org/10.1111/liv.14409. [Pubmed]
- 109. Racial and Ethnic Differences in Presentation and Outcomes of Hepatocellular Carcinoma. Clin Gastroenterol Hepatol. 2019; 17:551–59.e1. https://doi.org/10.1016/j.cgh.2018.05.039. [Pubmed]
- 110. Association of County-Level Vulnerability, Patient-Level Race/Ethnicity, and Receipt of Surgery for Early-Stage Hepatocellular Carcinoma. JAMA Surg. 2021; 156:197–99. https://doi.org/10.1001/jamasurg.2020.5554. [Pubmed]
- 111. Presentation, Management, and Outcomes Across the Rural-Urban Continuum for Hepatocellular Carcinoma. JNCI Cancer Spectr. 2021; 5:pkaa100. https://doi.org/10.1093/jncics/pkaa100. [Pubmed]
- 112. Genomic Analysis Revealed New Oncogenic Signatures in TP53-Mutant Hepatocellular Carcinoma. Front Genet. 2018; 9:2. https://doi.org/10.3389/fgene.2018.00002. [Pubmed]
