Genes & Cancer
Leveraging a powerful allogeneic dendritic cell line towards neoantigen-based cancer vaccines
Dalil Hannani1, Estelle Leplus1, Karine Laulagnier1, Laurence Chaperot2 and Joël Plumas1,2
1 PDC*line Pharma, Grenoble, France
2 R&D Laboratory, Etablissement Français du Sang Auvergne Rhône-Alpes (EFS AURA), Grenoble, France
Correspondence to: Joël Plumas, email: [email protected]
Keywords: cancer vaccine; neoantigens; plasmacytoid dendritic cells; immunotherapy
Received: August 25, 2022
Accepted: January 20, 2023
Published: January 30, 2023
Copyright: © 2023 Hannani et al. This is an open access article distributed under the terms of the Creative Commons Attribution License(CC BY 3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ABSTRACT
In recent years, immunotherapy has finally found its place in the anti-cancer therapeutic arsenal, even becoming standard of care as first line treatment for metastatic forms. The clinical benefit provided by checkpoint blockers such as anti-PD-1/PD-L1 in many cancers revolutionized the field. However, too many patients remain refractory to these treatments due to weak baseline anti-cancer immunity. There is therefore a need to boost the frequency and function of patients’ cytotoxic CD8+ cellular effectors by targeting immunogenic and tumor-restricted antigens, such as neoantigens using an efficient vaccination platform. Dendritic cells (DC) are the most powerful immune cell subset for triggering cellular immune response. However, autologous DC-based vaccines display several limitations, such as the lack of reproducibility and the limited number of cells that can be manufactured. Here we discuss the advantages of a new therapeutic vaccine based on an allogeneic Plasmacytoid DC cell line, which is easy to produce and represents a powerful platform for priming and expanding anti-neoantigen cytotoxic CD8+ T-cells.
INTRODUCTION
Due to the limited clinical benefit of anti-PD-1/PD-L1 therapy in many cancer indications, there is a renewed interest in therapeutic cancer vaccines to improve clinical responses. Indeed, one of the main explanations for resistance to these immune checkpoint inhibitors (ICI) is the absence of pre-existing anti-tumor immunity or the inadequacy of this immune response [1]. These therapeutic antibodies block the interaction between the inhibitory molecule PD-1 expressed on anti-tumor CD8+ T-cells and its ligand PD-L1, expressed by tumor cells. Their expected in vivo mechanism of action is thus to unleash the cytotoxic activity of anti-tumor effectors [2]. In addition, different reports describing the effect of the treatment of patients with ICIs in a neo-adjuvant setting strongly suggested that reinforcing the patient’s own immune system led to the eradication of tumor cells, as evidenced by major or complete pathological responses [3–9]. Therefore, it is becoming increasingly clear that the combination of ICIs with therapeutic cancer vaccines that aimed at priming or enhancing anti-tumor CD8+ T-cell effectors could increase the efficacy of each treatment used separately [10–12].
Neoantigens as a source of tumor antigens for cancer immunotherapies
Among several potential tumor antigens that can be targeted by the immune system, neoantigens (NeoAgs) appear very attractive because they are tumor cell-specific proteins and unknown to the immune system (i.e., there is no pre-existing central immune tolerance) [13, 14]. NeoAgs were initially described as the result of non-synonymous somatic mutations [14], but they can also be derived from many other genomic abnormalities in the transcriptional and translational process leading to the synthesis of abnormal proteins [15–23]. Interestingly, the frequency of tumor somatic mutations correlates with objective response rates to ICIs in many cancers [24, 25]. Thus, these single nucleotide variants may serve as neoantigens recognized by the immune system, leading to tumor cell death mediated by NeoAg-specific CD8+ T-cells. Very recently, the number and frequency of NeoAg-specific CD8+ T-cells were confirmed to be associated with the clinical outcome of adoptive cell therapy with tumor-infiltrating lymphocytes (TIL) by using an elegant approach [26]. Interestingly, it was also suggested recently that the expansion and activation of NeoAg-specific CD8+ T-cells are associated with the response to ICIs in patients with metastatic urothelial carcinoma [27]. However, despite the considerable number of diverse genomic abnormalities, very few candidates are considered as “good” NeoAgs. This is due to the highly selective molecular machinery allowing the presentation of an immunogenic peptide derived from NeoAgs to the immune system through HLA class I molecules expressed by tumor cells [15, 23]. Recent significant developments in algorithms and deep machine learning have provided opportunities to identify few NeoAgs in the majority of patients, especially in cancers induced by mutagens or DNA mismatch repair [16, 17]. This is probably why therapeutic NeoAg-based cancer vaccines were first developed in melanoma [28–30]. The availability of resected tumors has led to develop vaccines also in glioblastoma, non-small-cell lung cancer (NSCLC), bladder, gastrointestinal, colorectal, urothelial, and pancreatic cancers [31–41]. All studies, except two [36, 37], have so far used private NeoAgs, i.e., identified in a single patient. Most of the clinical studies published are still in phase I or Phase I/II and despite the combination with ICIs, these vaccine approaches are not yet validated clinically.
From an immunological point of view, it is quite surprising that many studies used a vaccine regimen based on local injections of long peptides combined with adjuvants [29, 31–33, 36, 37, 41]. Indeed, these approaches were known to be rather suboptimal to prime and stimulate anti-tumor CD8+ T-cells, and may even generate tolerogenic responses [42–46]. As a result, very weak NeoAg-specific CD8+ T-cell responses have been obtained from patients, in contrast to NeoAg-specific CD4+ T-cells which are not the main effectors of anti-tumor immune response. Indeed, except in rare cases, CD4+ T-cells are not cytotoxic and cannot kill tumor cells due to the lack of expression of HLA class II molecules by tumor cells. RNA-based approaches have also been tested with no significant change in the nature and the amplitude of the anti-tumor response [30, 34]. However, Moderna and Merck have recently reported results on melanoma that will deserve attention when published. The use of adenoviral-based platform has been recently described with some interesting results in few patients [39, 40]. By contrast, the use of mature dendritic cells (DC) loaded with short peptides derived from NeoAgs has demonstrated strong expansions of cytotoxic CD8+ T-cells for many NeoAgs in all melanoma patients tested [28].
Dendritic cells are essential for the induction of anti-tumor response
Dendritic cells are perfectly equipped to process and present tumor antigen-derived peptides to naive CD8+ T-cells in lymphoid organs, transforming them into effector memory cells capable of reaching to the tumor site and killing tumor cells [47, 48]. They are also very effective in reactivating circulating and tissue-resident anti-tumor memory T-cells [47]. Dendritic cells therefore appear to be of great interest for the development of a cancer vaccine based on NeoAgs, as they directly and efficiently stimulate the appropriate anti-tumor effector cells after injection, avoiding any induction of tolerance [49, 50]. However, to date, given that the main antigen-presenting platforms have used autologous DCs, they have faced major challenges: the cost of manufacturing, reproducibility, feasibility, the availability of sufficient drug product, the suboptimal efficacy of the product, the difficulty of establishing quality control of immune activity, and the heterogeneity of clinical trials since all patients were treated with a different drug product [51]. Except in prostate cancer [52] and very recently in glioblastoma [53], autologous DC-based vaccines have not yet proven their efficiency [54]. Interestingly, numerous issues can be solved using allogeneic dendritic cells [55]. Indeed, allogeneic DCs can be easily manufactured, as the cell source is independent of patients. In addition, the cell drug product is shortly available for the patients when they are enrolled and its potency to stimulate anti-tumor CD8+ T-cells can be checked before infusion.
Allogeneic plasmacytoid dendritic cells represent an efficient vaccination platform
We have developed a novel approach using an allogeneic plasmacytoid dendritic cell (PDC) line as an antigen-presentation platform showing great potency to prime and expand viral or tumor-specific CD8+ T cells in vitro and in vivo in a humanized mouse model [55–65]. This off-the-shelf product is scalable, versatile, cost-effective, and guarantees the homogeneity of treatment and clinical results as the same product is used for all patients. This PDC platform, named PDC*vac, was first evaluated with shared tumor-associated antigens in the treatment of melanoma with encouraging results [66]. This first-in-human phase I clinical trial demonstrated PDC*vac safety and biological activity since it primed and expanded anti-tumor CD8+ T-cells in patients. Moreover, we have shown the in vitro synergy of PDC*vac with anti-PD-1 drug product leading to the improved expansion of anti-tumor CD8+ T-cells from metastatic melanoma patients. The PDC*vac platform adapted to lung cancer patients (PDC*lung01 product) is currently being evaluated in the treatment of metastatic squamous and non-squamous lung cancer patients in combination with anti-PD-1 antibody (NCT03970746). The preliminary results of this phase I/II are very encouraging in terms of safety, biological, and clinical activities [67].
Given the afore-mentioned advantages of NeoAgs in vaccine approaches, we have exploited the PDC*vac platform in order to activate NeoAg-specific immune response using the same methodology as previously described [58, 66].
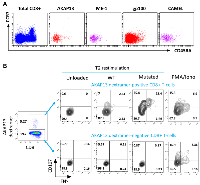
We have performed in vitro experiments showing that this new product named PDC*neo can effectively prime and expand NeoAg-specific CD8+ T-cells. As a proof of concept, PDC*line cells were loaded with two NeoAgs (ME-1 and AKAP13, Table 1) already described in melanoma and lung cancer patients [28, 68] and two commonly shared tumor-associated antigens as positive controls (gp100, CAMEL). Loaded PDC*line was then cultured with purified healthy donors’ CD8+ T-cells for 3 weeks before detecting specific T-cells with multimer tools (Figure 1). In such experiments, we used CD8+ T-cells purified from healthy donors because they were naive, and thus never encountered NeoAgs. As a consequence, the basal circulating precursor frequencies were expected extremely low (less or equal to 1/1,000,000 in total CD8+ population). However, after weekly stimulations of these rare naive cells with PDC*neo product, a sizeable expansion of antigen-specific CD8+ T-cells was observed as soon as 7 days of co-culture, followed by a powerful expansion at day 21 (Figure 1A and 1B). Indeed, the absolute number of antigen-specific T-cells highly increases from D7 to D21 for both ME-1 and AKAP13. (Figure 1C). As expected, CAMEL- and gp100-specific T-cells were also massively primed and expanded confirming the potency of PDC*line cells (Figure 1C).
Interestingly, after 21 days of culture with PDC*vac, all antigen-specific T-cells displayed an effector/memory phenotype (CCR7neg and CD45RAneg; Figure 2A). Moreover, the NeoAg-specific CD8+ T-cells induced by PDC*vac presented functional activity as shown by the expression of CD107 and IFNγ upon stimulation (Figure 2B). Noteworthy, these cells were specific to the mutated form of the neopeptide as they did not react against the wild-type peptide.
Altogether, these data demonstrate that PDC*vac represents an interesting tool for assessing the immunogenicity of neo-epitopes in vitro, as well as a powerful vaccine platform for NeoAg-based cancer vaccines. Indeed, PDC*line is a highly potent professional antigen-presenting cell that migrates in lymph nodes and tissues (unpublished data) to directly stimulate peptide-specific CD8+ T-cells. The allogeneic context may bring supplementary activation signal for the immune system. As PDC*line cells are loaded with short peptides, there is no need of antigen transcription, translation, and processing since the peptides are directly loaded on and presented by surface HLA molecules. Finally, the direct presentation of peptides by the dendritic cells themselves avoids any unwanted tolerance induction.
CONCLUSIONS
NeoAgs appear attractive candidates to induce specific anti-tumor responses in cancer patients, on top of classical tumor-associated antigens and in association with ICIs. A potent dendritic cell product such as PDC*neo represents a valuable platform to develop NeoAg-based cancer vaccines (Figure 3). We strongly believe that this new delivery technology based on potent PDC*line cells can induce a robust anti-NeoAg CD8+ T-cell immune response for the benefit of patients and could reshape the landscape of NeoAg-based cancer vaccines.
Author contributions
D. Hannani and E. Leplus performed experiments; J. Plumas supervised the study and wrote the article; K. Laulagnier, D. Hannani, and L. Chaperot reviewed the article.
CONFLICTS OF INTEREST
Joel Plumas is Chief Scientific Officer of PDC*line Pharma. Dalil Hannani, Estelle Leplus, and Karine Laulagnier are employees of PDC*line Pharma.
FUNDING
This research was funded by Etablissement Français du Sang (EFS AURA), the Wallon Region and by PDC*line Pharma.

- 1. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014; 515:568–71. https://doi.org/10.1038/nature13954. [Pubmed]
- 2. Dendritic Cells and Programmed Death-1 Blockade: A Joint Venture to Combat Cancer. Front Immunol. 2018; 9:394. https://doi.org/10.3389/fimmu.2018.00394. [Pubmed]
- 3. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020; 367:eaax0182. https://doi.org/10.1126/science.aax0182. [Pubmed]
- 4. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019; 25:477–86. https://doi.org/10.1038/s41591-018-0337-7. [Pubmed]
- 5. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 2018; 24:1649–54. https://doi.org/10.1038/s41591-018-0197-1. [Pubmed]
- 6. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med. 2019; 25:454–61. https://doi.org/10.1038/s41591-019-0357-y. [Pubmed]
- 7. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018; 24:1655–61. https://doi.org/10.1038/s41591-0180198-0. [Pubmed]
- 8. Improved Efficacy of Neoadjuvant Compared to Adjuvant Immunotherapy to Eradicate Metastatic Disease. Cancer Discov. 2016; 6:1382–99. https://doi.org/10.1158/2159-8290.CD-16-0577. [Pubmed]
- 9. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med. 2021; 27:504–14. https://doi.org/10.1038/s41591-020-01224-2. [Pubmed]
- 10. Therapeutic cancer vaccines. Nat Rev Cancer. 2021; 21:360–78. https://doi.org/10.1038/s41568-021-00346-0. [Pubmed]
- 11. Integrating Next-Generation Dendritic Cell Vaccines into the Current Cancer Immunotherapy Landscape. Trends Immunol. 2017; 38:577–93. https://doi.org/10.1016/j.it.2017.05.006. [Pubmed]
- 12. Is there a role for therapeutic cancer vaccines in the age of checkpoint inhibitors? Hum Vaccin Immunother. 2017; 13:528–32. https://doi.org/10.1080/21645515.2016.1244149. [Pubmed]
- 13. The makings of a tumor rejection antigen. Immunity. 1999; 11:263–70. https://doi.org/10.1016/s1074-7613(00)80101-6. [Pubmed]
- 14. Neoantigens in cancer immunotherapy. Science. 2015; 348:69–74. https://doi.org/10.1126/science.aaa4971. [Pubmed]
- 15. Alternative tumour-specific antigens. Nat Rev Cancer. 2019; 19:465–78. https://doi.org/10.1038/s41568-019-0162-4. [Pubmed]
- 16. Neoantigen prediction and computational perspectives towards clinical benefit: recommendations from the ESMO Precision Medicine Working Group. Ann Oncol. 2020; 31:978–90. https://doi.org/10.1016/j.annonc.2020.05.008. [Pubmed]
- 17. Challenges in neoantigen-directed therapeutics. Cancer Cell. 2023; 41:15–40. https://doi.org/10.1016/j.ccell.2022.10.013. [Pubmed]
- 18. A library of Neo Open Reading Frame peptides (NOPs) as a sustainable resource of common neoantigens in up to 50% of cancer patients. Sci Rep. 2019; 9:6577. https://doi.org/10.1038/s41598-019-42729-2. [Pubmed]
- 19. Shared Immunogenic Poly-Epitope Frameshift Mutations in Microsatellite Unstable Tumors. Cell. 2020; 183:1634–49.e17. https://doi.org/10.1016/j.cell.2020.11.004. [Pubmed]
- 20. RNA editing derived epitopes function as cancer antigens to elicit immune responses. Nat Commun. 2018; 9:3919. https://doi.org/10.1038/s41467-018-06405-9. [Pubmed]
- 21. Cancer-Specific Splicing Changes and the Potential for Splicing-Derived Neoantigens. Cancer Cell. 2018; 34:181–83. https://doi.org/10.1016/j.ccell.2018.07.008. [Pubmed]
- 22. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol. 2017; 18:1009–21. https://doi.org/10.1016/S1470-2045(17)30516-8. [Pubmed]
- 23. Exploiting non-canonical translation to identify new targets for T cell-based cancer immunotherapy. Cell Mol Life Sci. 2018; 75:607–21. https://doi.org/10.1007/s00018-017-2628-4. [Pubmed]
- 24. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer. 2017; 17:209–22. https://doi.org/10.1038/nrc.2016.154. [Pubmed]
- 25. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015; 348:124–28. https://doi.org/10.1126/science.aaa1348. [Pubmed]
- 26. Neoantigen-reactive CD8+ T cells affect clinical outcome of adoptive cell therapy with tumor-infiltrating lymphocytes in melanoma. J Clin Invest. 2022; 132:e150535. https://doi.org/10.1172/JCI150535. [Pubmed]
- 27. Neoantigen-specific CD8 T cell responses in the peripheral blood following PD-L1 blockade might predict therapy outcome in metastatic urothelial carcinoma. Nat Commun. 2022; 13:1935. https://doi.org/10.1038/s41467-022-29342-0. [Pubmed]
- 28. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015; 348:803–8. https://doi.org/10.1126/science.aaa3828. [Pubmed]
- 29. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017; 547:217–21. https://doi.org/10.1038/nature22991. [Pubmed]
- 30. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017; 547:222–26. https://doi.org/10.1038/nature23003. [Pubmed]
- 31. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019; 565:240–45. https://doi.org/10.1038/s41586-018-0810-y. [Pubmed]
- 32. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019; 565:234–39. https://doi.org/10.1038/s41586-018-0792-9. [Pubmed]
- 33. A Phase Ib Trial of Personalized Neoantigen Therapy Plus Anti-PD-1 in Patients with Advanced Melanoma, Non-small Cell Lung Cancer, or Bladder Cancer. Cell. 2020; 183:347–62.e24. https://doi.org/10.1016/j.cell.2020.08.053. [Pubmed]
- 34. mRNA vaccine-induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J Clin Invest. 2020; 130:5976–88. https://doi.org/10.1172/JCI134915. [Pubmed]
- 35. A Phase Ib Study of the Combination of Personalized Autologous Dendritic Cell Vaccine, Aspirin, and Standard of Care Adjuvant Chemotherapy Followed by Nivolumab for Resected Pancreatic Adenocarcinoma-A Proof of Antigen Discovery Feasibility in Three Patients. Front Immunol. 2019; 10:1832. https://doi.org/10.3389/fimmu.2019.01832. [Pubmed]
- 36. Mass cytometry detects H3.3K27M-specific vaccine responses in diffuse midline glioma. J Clin Invest. 2020; 130:6325–37. https://doi.org/10.1172/JCI140378. [Pubmed]
- 37. A vaccine targeting mutant IDH1 in newly diagnosed glioma. Nature. 2021; 592:463–68. https://doi.org/10.1038/s41586-021-03363-z. [Pubmed]
- 38. Personalized neoantigen vaccine prevents postoperative recurrence in hepatocellular carcinoma patients with vascular invasion. Mol Cancer. 2021; 20:164. https://doi.org/10.1186/s12943-021-01467-8. [Pubmed]
- 39. Adenoviral-based vaccine promotes neoantigen-specific CD8(+) T cell stemness and tumor rejection. Sci Transl Med. 2022; 14:eabo7604. https://doi.org/10.1126/scitranslmed.abo7604. [Pubmed]
- 40. Individualized, heterologous chimpanzee adenovirus and self-amplifying mRNA neoantigen vaccine for advanced metastatic solid tumors: phase 1 trial interim results. Nat Med. 2022; 28:1619–29. https://doi.org/10.1038/s41591-022-01937-6. [Pubmed]
- 41. Personalized neoantigen vaccine NEO-PV-01 with chemotherapy and anti-PD-1 as first-line treatment for non-squamous non-small cell lung cancer. Cancer Cell. 2022; 40:1010–26.e11. https://doi.org/10.1016/j.ccell.2022.08.003. [Pubmed]
- 42. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016; 17:822–35. https://doi.org/10.1016/S1470-2045(16)00099-1. [Pubmed]
- 43. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2014; 15:59–68. https://doi.org/10.1016/S1470-2045(13)70510-2. [Pubmed]
- 44. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open-label, randomised, phase 3 trial. Lancet Oncol. 2014; 15:829–40. https://doi.org/10.1016/S1470-2045(14)70236-0. [Pubmed]
- 45. Persistent antigen at vaccination sites induces tumor-specific CD8⁺ T cell sequestration, dysfunction and deletion. Nat Med. 2013; 19:465–72. https://doi.org/10.1038/nm.3105. [Pubmed]
- 46. A leukemic plasmacytoid dendritic cell line, PMDC05, with the ability to secrete IFN-alpha by stimulation via Toll-like receptors and present antigens to naïve T cells. Leuk Res. 2009; 33:1224–32. https://doi.org/10.1016/j.leukres.2009.03.047. [Pubmed]
- 47. Immunobiology of dendritic cells. Annu Rev Immunol. 2000; 18:767–811. https://doi.org/10.1146/annurev.immunol.18.1.767. [Pubmed]
- 48. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012; 30:1–22. https://doi.org/10.1146/annurev-immunol-100311-102839. [Pubmed]
- 49. Dendritic Cell-Based Immunotherapy in Lung Cancer. Front Immunol. 2020; 11:620374. https://doi.org/10.3389/fimmu.2020.620374. [Pubmed]
- 50. Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer. 2019; 18:128. https://doi.org/10.1186/s12943-019-1055-6. [Pubmed]
- 51. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020; 20:7–24. https://doi.org/10.1038/s41577-019-0210-z. [Pubmed]
- 52. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010; 363:411–22. https://doi.org/10.1056/NEJMoa1001294. [Pubmed]
- 53. Association of Autologous Tumor Lysate-Loaded Dendritic Cell Vaccination With Extension of Survival Among Patients With Newly Diagnosed and Recurrent Glioblastoma: A Phase 3 Prospective Externally Controlled Cohort Trial. JAMA Oncol. 2023; 9:112–21. https://doi.org/10.1001/jamaoncol.2022.5370. [Pubmed]
- 54. Trial watch: dendritic cell vaccination for cancer immunotherapy. Oncoimmunology. 2019; 8:e1638212. https://doi.org/10.1080/2162402X.2019.1638212. [Pubmed]
- 55. Harnessing dendritic cells for innovative therapeutic cancer vaccines. Curr Opin Oncol. 2022; 34:161–68. https://doi.org/10.1097/CCO.0000000000000815. [Pubmed]
- 56. A novel cancer vaccine strategy based on HLA-A*0201 matched allogeneic plasmacytoid dendritic cells. PLoS One. 2010; 5:e10458. https://doi.org/10.1371/journal.pone.0010458. [Pubmed]
- 57. HLA-A(*)0201(+) plasmacytoid dendritic cells provide a cell-based immunotherapy for melanoma patients. J Invest Dermatol. 2012; 132:2395–406. https://doi.org/10.1038/jid.2012.152. [Pubmed]
- 58. Engineering a Human Plasmacytoid Dendritic Cell-Based Vaccine to Prime and Expand Multispecific Viral and Tumor Antigen-Specific T-Cells. Vaccines (Basel). 2021; 9:141. https://doi.org/10.3390/vaccines9020141. [Pubmed]
- 59. Induction of antiviral cytotoxic T cells by plasmacytoid dendritic cells for adoptive immunotherapy of posttransplant diseases. Am J Transplant. 2011; 11:2613–26. https://doi.org/10.1111/j.1600-6143.2011.03722.x. [Pubmed]
- 60. pDCs efficiently process synthetic long peptides to induce functional virus- and tumour-specific T-cell responses. Eur J Immunol. 2014; 44:2880–92. https://doi.org/10.1002/eji.201444588. [Pubmed]
- 61. Preparation of purified lymphoma cells suitable for therapy. Cytotherapy. 2004; 6:235–43. https://doi.org/10.1080/14653240410006059. [Pubmed]
- 62. Plasmacytoid dendritic cells induce efficient stimulation of antiviral immunity in the context of chronic hepatitis B virus infection. Hepatology. 2012; 56:1706–18. https://doi.org/10.1002/hep.25879. [Pubmed]
- 63. Highly efficient transduction of human plasmacytoid dendritic cells without phenotypic and functional maturation. J Transl Med. 2009; 7:10. https://doi.org/10.1186/1479-5876-7-10. [Pubmed]
- 64. Virosome-mediated delivery of tumor antigen to plasmacytoid dendritic cells. Vaccine. 2007; 25:3913–21. https://doi.org/10.1016/j.vaccine.2007.01.101. [Pubmed]
- 65. Whole lymphoma B cells allow efficient cross-presentation of antigens by dendritic cells. Cytotherapy. 2008; 10:642–49. https://doi.org/10.1080/14653240802317647. [Pubmed]
- 66. An innovative plasmacytoid dendritic cell line-based cancer vaccine primes and expands antitumor T-cells in melanoma patients in a first-in-human trial. Oncoimmunology. 2020; 9:1738812. https://doi.org/10.1080/2162402X.2020.1738812. [Pubmed]
- 67. 1176P Open-label, dose escalation, phase I/II study to assess safety, tolerability, immunogenicity and preliminary clinical activity of the therapeutic cancer vaccine PDC*lung01 with or without anti-programmed death-1 (PD-1) treatment in patients with non-small cell lung cancer (NSCLC).Ann Oncol. 2022; 33:S1086. https://doi.org/10.1016/j.annonc.2022.07.1299.
- 68. High frequency of cytolytic T lymphocytes directed against a tumor-specific mutated antigen detectable with HLA tetramers in the blood of a lung carcinoma patient with long survival. Cancer Res. 2001; 61:3718–24. [Pubmed]
