Genes & Cancer
A novel t (5; 17) (q35; q21) associated with t (8; 21) (q22; q22) in a patient with acute myeloid leukemia: case report and review of literature
Kmira Zahra1, Wided Cherif1, Gereisha Ahmed2, Haifa Regaieg1, Ben Sayed Nesrine1, Monia Zaier1, Wided Mootamri2, Yosra Ben Youssef1, Nejia Brahem2, Halima Sennana3 and Abderrahim Khelif1
1Department of Clinical Hematology, Farhat Hached University Hospital-Sousse-Tunisia, Sousse 4081, Tunisia
2Department of Cytology, Farhat Hached University Hospital-Sousse-Tunisia, Sousse 4081, Tunisia
3Department of Cytogenetics, Farhat Hached University Hospital-Sousse-Tunisia, Sousse 4081, Tunisia
Correspondence to: Kmira Zahra, email: [email protected]
Keywords: acute myeloid leukemia; T (8; 21) (q22; q22); T (5; 17) (q35; q21); NPM1/RARA; allogenic stem cells transplantation
Received: April 01, 2023
Accepted: June 14, 2023
Published: June 28, 2023
Copyright: © 2023 Zahra et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ABSTRACT
The t (8; 21) (q22; q22) with the resulting RUNX1- RUNX1T1 rearrangement is one of the most common cytogenetic abnormalities in acute myeloid leukemia (AML). It is associated with favorable prognosis. The t (5; 17) (q35; q21) is an uncommon translocation, fuses the gene for the nucleophosmin (NPM) to the retinoic acid receptor α(RARA) and was described essentially in acute promyelocytic leukemia (APL) variant. We present the case of a 19-year-old male patient who developed an AML with t (8; 21) (q22; q22) associated to t (5; 17) (q35; 21). Morphology and immunophenotype of the leukemic cells were compatible with AML. The patient received chemotherapy based on cytarabine and anthracycline without all-trans retinoic acid (ATRA) followed by allogenic stem cell transplantation in first remission. To the best of our knowledge, this is the first report of an association between a rare translocation t (5; 17) and t (8; 21) in AML. In this report, we will discuss the prognosis of this association as well as the treatment.
INTRODUCTION
Acute myeloid leukemia (AML) is the most common acute leukemia in adults and counts for about 80% of all cases [1]. Cytogenetic analysis is the most important diagnostic tool for determining prognosis. In fact, numerous recurrent karyotype abnormalities have been discovered in AML. Commonly observed chromosomal abnormalities in AML are t (8; 21), t (15; 17), inv (16), which are associated with higher rates of complete remission and event free survival [2]. T (5; 17) in AML is exceptional, described in few cases in acute promyelocytic leukemia (APL) variant [3, 4].
Here we report an unusual association of t (5; 17) with t (8; 21) in AML and we try to discuss the prognosis of this association and then the treatment.
CASE REPORT
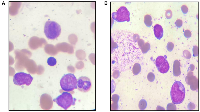
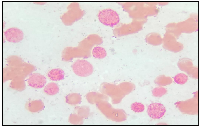
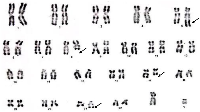
A 19-year-old male patient presented in December 2017 with a one month history of fever and asthenia. Physical examination was normal; particularly absence of lymphadenopathy and splenomegaly. The white blood cell count was 42 000/mm3 consisting of 10% neutrophils, 10% lymphocytes, and 80% blasts. The hemoglobin was 6.9 g/dl and the platelet count was 10 000/mm3. Coagulation test showed disseminated intravascular coagulation (DIC) with a prolonged activated partial thromboplastin time of 45 sec, prolonged prothrombin time and an increased d-dimers concentration of 1460 mg/mmol. Bone marrow analysis revealed increased blasts (98%) with typical morphology of AML type 1 according to the FAB classification: The cytoplasm is moderately basophilic, sometimes containing fine granules and/or Auer bodies (Figures 1 and 2). Flow cytometric analysis showed that the leukemic cells were positive for CD13, CD33, CD117, CD34, and MPO. Cytogenetic study of the bone marrow cells revealed in all the AML cells a 46, XY, t (8, 21) (q22; q22) with t (5, 17) (q35, q21) [20 mitoses] Karyotype (Figure 3). RT-PCR analysis performed on the bone marrow aspirate revealed the presence of AML/ETO fusion transcript (Rearrangement of RUNX1-RUNX1T1). Due to a shortage of probe, the research of the rearrangement NPM1-RARA was not performed. The patient has reached cytologic and cytogenetic remission after undergoing induction therapy with cytarabine (200 mg/m²) for 7 days associated with idarubicin (12 mg/m²) for 3 days. The all-trans retinoic acid (ATRA) was not received and the DIC was managed by Fresh Frozen Plasma (FFP) transfusion. After that, the patient received two cycles of consolidations, the first associated cytarabine 200 mg/m² × 7 days, daunorubicin 60 mg/m2 × 3 days and etoposide 100 mg/m2 × 5 days and the second consisted of high dose cytarabine 6 g/m² for 3 days. The bone marrow evaluation revealed persistent cytologic and cytogenetic remission but a positive minimal residual disease (MRD) indicating the allogeneic transplantation of stem cells. RT-PCR for RUNX1- RUNX1T1 before the allograft was negative. The patient is so far alive in persistent complete remission.
DISCUSSION
The t (8; 21), is reported to be found in 5–10% of all AML cases [5, 6]. On the morphological level, this entity is characterized by the presence of large blasts having basophilic cytoplasm associated with numerous azurophilic granules and a perinuclear clearing. Auer bodies are also commonly reported and may be detected in blasts or immature neutrophils. Bone marrow examination frequently reveals granulocytic maturation phases to promyelocytes, myelocytes and mature neutrophils that could sometimes be associated with dysplasia features [7]. The t (5; 17) in AML is exceptional, described in few cases in APL variant [3, 4]. The morphology usually shows a Hypergranular and hypogranular bilobed promyelocytes; absence of Auer rods; typical microspeckled pattern with anti-RARa antibodies [3, 4]. In our patient, this unusual association of t (5; 17) to t (8; 21) doesn’t affect the morphology of the cells and the bone marrow aspiration shows blasts with typical morphology of AML type1 according to the FAB classification: The cytoplasm is moderately basophilic, sometimes containing fine granules and/or Auer bodies.
On the immunological level of AML with t (8; 21), the cells, usually tend to express the following markers: high levels of CD34, HLA-DR, myeloperoxidase (MPO) and CD13 (7). A relative weak expression of CD33 was found, and aberrant lymphoid associated markers (CD19, CD56, cCD79a, Pax5) expression was reported with variable prognosis implications [8–10]. Moreover, on flow cytometry assessment in our case, we concluded to the expression of the following antigens CD13+, CD33, CMPO+, CD117+, CD34+ which are typically described with t (8; 21) and therefore, this unusual association of t (5, 17) to t (8; 21) doesn’t affect the immunological phenotype of the cells.
In few cases with a morphological diagnosis of APL, patients have variant chromosome translocations, which fuse RARA gene with partner genes other than PML, such as in the variant translocation t (5; 17) (q35; q21) that fuses the N-terminus of nucleophosmin (NPM1) gene at 5q35 to the retinoic acid receptor alpha at 17q21 [11, 12]. In our case, NPM1-RARA not realized for lack of the specific probe.
In APL with the classic reciprocal translocation t (15; 17) (q22; q21) which is characterized by fusion gene transcript PML-RAR-alpha, patients are responsive to differentiation treatment with all-trans retinoic acid (ATRA) [13]. In our case, the research of NPM1-RARA was not possibly due to scarcity of probes and therefore the patient didn’t receive the ATRA.
Unbalanced translocation der (5; 17) resulting in a TP53 loss [14], whole-arm translocation of der (5; 17)(p10; q10) with concurrent TP53 mutations [15] and reccuring abnormality dic (5; 17) associated also with mutations of TP53 [16] were described in AML. The association with P53 abnormalities was not tested in our patient.
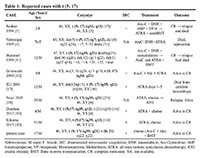
Up to now only 7 cases with balanced t (5; 17) (q35; q12-21) translocation and the underlying NPM1/RARA fusion have been identified, remission obtained with chemotherapy associated or not with ATRA. The different cases were detailed in Table 1.
In two studies [3, 11] (Table 1), the ATRA associated with chemotherapy was used in second line therapy leading to short remission in patients with t (5; 17) suggesting its beneficial effect in first line therapy. Chemotherapy with differentiation therapy has been used in the majority of cases of the t (5; 17) in the literature [12, 19, 20]. The use of differentiation therapy alone (ATRA, Arsenic trioxide) has not been reported. While patients’ response to ATRA alone is difficult to assess since chemotherapy was part of the induction treatment, the differentiation effects of ATRA probably led to remission in these patients. Indeed, Redner and colleagues have demonstrated that in short term culture systems, cells bearing the t (5; 17) translocation terminally differentiate in response to ATRA [21]. Our case is very interesting describing, to our knowledge, the first case of t (8; 21) associated with t (5; 17). Bone marrow examination revealed positive MRD by flow cytometry (positive at threshold 10−4) after consolidation which led to the decision to recommend allogenic stem cell transplantation. Our patient didn’t receive ATRA in combination with chemotherapy (Marketing Authorization in Tunisia for only APL) and perhaps if he received it, he would have negative residual disease after the consolidation and therefore no indication for allogenic stem cell transplantation. The prognosis is difficult to assess, and further accumulation of data on such cases is desirable in order to be able to conclude.
CONCLUSION
We have described a patient with a novel balanced t (5; 17) (q35; q21) translocation emerging with t (8; 21) in AML. To our knowledge, this additional chromosomal aberration has not yet been described. This aberration is thought to play a crucial role in prognosis. The efficacy of ATRA is controversial. Therefore, further accumulation of data on such cases is warranted to enable more comprehensive and conclusive evaluations.
Data availability statement
The dataset of the current study is available from the corresponding author upon motivated request.
Author contributions
Kmira Zahra, Wided Cherif, Gereisha Ahmed: wrote the main manuscript. Wided Mootamri, Ben Sayed Nesrine, Nejia Brahem, Halima Sennana: prepared the figures. Haifa Regaieg, Monia Zaier, Yosra Ben Youssef, Abderrahim Khelif: revised the manuscript.
CONFLICTS OF INTEREST
Authors have no conflicts of interest to declare.
- 1. Patterns of leukemia incidence in the United States by subtype and demographic characteristics, 1997-2002. Cancer Causes Control. 2008; 19:379–90. https://doi.org/10.1007/s10552-007-9097-2. [Pubmed]
- 2. ‘Acute myeloid leukemia: a comprehensive review and 2016 update’. Blood Cancer J. 2016; 6:e441. https://doi.org/10.1038/bcj.2016.50. [Pubmed]
- 3. The t(5;17) variant of acute promyelocytic leukemia expresses a nucleophosmin-retinoic acid receptor fusion. Blood. 1996; 87:882–86. [Pubmed]
- 4. Unbalanced translocation t(5;17) in an typical acute promyelocytic leukemia. Genes Chromosomes Cancer. 1995; 14:307–12. https://doi.org/10.1002/gcc.2870140410. [Pubmed]
- 5. Prognostic impact of acute myeloid leukemia classification. Importance of detection of recurring cytogenetic abnormalities and multilineage dysplasia on survival. Am J Clin Pathol. 2003; 119:672–80. https://doi.org/10.1309/EM7K-CQR4-GLMH-RCX4. [Pubmed]
- 6. Cytogenetic profile in de novo acute myeloid leukemia with FAB subtypes M0, M1, and M2: a study based on 652 cases analyzed with morphology, cytogenetics, and fluorescence in situ hybridization. Cancer Genet Cytogenet. 2004; 155:47–56. https://doi.org/10.1016/j.cancergencyto.2004.03.008. [Pubmed]
- 7. Morphological subtyping of acute myeloid leukemia with maturation (AML-M2): homogeneous pink-colored cytoplasm of mature neutrophils is most characteristic of AML-M2 with t(8;21). Leukemia. 1997; 11:651–55. https://doi.org/10.1038/sj.leu.2400618. [Pubmed]
- 8. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010; 116:354–65. https://doi.org/10.1182/blood-2009-11-254441. [Pubmed]
- 9. Surface markers in adult acute myeloblastic leukemia: correlation of CD19+, CD34+ and CD14+/DR--phenotypes with shorter survival. Groupe d’Etude Immunologique des Leucémies (GEIL). Leukemia. 1992; 6:393–99. [Pubmed]
- 10. A correlation study of immunophenotypic, cytogenetic, and clinical features of 180 AML patients in China. Cytometry B Clin Cytom. 2008; 74:25–29. https://doi.org/10.1002/cyto.b.20368. [Pubmed]
- 11. Deregulation of NPM and PLZF in a variant t(5;17) case of acute promyelocytic leukemia. Oncogene. 1999; 18:633–41. https://doi.org/10.1038/sj.onc.1202357. [Pubmed]
- 12. Characterization of acute promyelocytic leukemia cases lacking the classic t(15;17): results of the European Working Party. Groupe Français de Cytogénétique Hématologique, Groupe de Français d’Hematologie Cellulaire, UK Cancer Cytogenetics Group and BIOMED 1 European Community-Concerted Action “Molecular Cytogenetic Diagnosis in Haematological Malignancies”. Blood. 2000; 96:1297–308. [Pubmed]
- 13. Acute promyelocytic leukemia current treatment algorithms. Blood Cancer J. 2021; 11:123. https://doi.org/10.1038/s41408-021-00514-3. [Pubmed]
- 14. Unbalanced translocation der(5;17) resulting in a TP53 loss as recurrent aberration in myelodysplastic syndrome and acute myeloid leukemia with complex karyotype. Genes Chromosomes Cancer. 2021; 60:452–57. https://doi.org/10.1002/gcc.22938. [Pubmed]
- 15. Whole-arm translocation of der(5;17)(p10;q10) with concurrent TP53 mutations in acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS): A unique molecular-cytogenetic subgroup. Cancer Genet. 2016; 209:205–14. https://doi.org/10.1016/j.cancergen.2016.04.001. [Pubmed]
- 16. dic(5;17): a recurring abnormality in malignant myeloid disorders associated with mutations of TP53. Genes Chromosomes Cancer. 1997; 20:282–91. https://doi.org/10.1002/(sici)1098-2264(199711)20:3<282::aid-gcc9>3.0.co;2-z. [Pubmed]
- 17. Molecular cytogenetic characterization and clinical relevance of additional, complex and/or variant chromosome abnormalities in acute promyelocytic leukemia. Leukemia. 2001; 15:1359–68. https://doi.org/10.1038/sj.leu.2402205. [Pubmed]
- 18. Molecular and cytogenetic characterization of a new case of t(5;17)(q35;q21) variant acute promyelocytic leukemia. Leukemia. 2005; 19:470–72. https://doi.org/10.1038/sj.leu.2403645. [Pubmed]
- 19. Acute promyelocytic leukemia following aleukemic leukemia cutis harboring NPM/RARA fusion gene. Pediatr Blood Cancer. 2012; 59:959–60. https://doi.org/10.1002/pbc.24199. [Pubmed]
- 20. A new transcriptional variant and small azurophilic granules in an acute promyelocytic leukemia case with NPM1/RARA fusion gene. Int J Hematol. 2015; 102:713–18. https://doi.org/10.1007/s12185-015-1857-2. [Pubmed]
- 21. Differentiation of t(5;17) variant acute promyelocytic leukemic blasts by all-trans retinoic acid. Leukemia. 1997; 11:1014–16. https://doi.org/10.1038/sj.leu.2400661. [Pubmed]
