Genes & Cancer
The apoptosis associated tyrosine kinase gene is frequently hypermethylated in human cancer and is regulated by epigenetic mechanisms
Tanja Haag1, Christina E. Herkt1, Sara K. Walesch1, Antje M. Richter1 and Reinhard H. Dammann1
1 Institute for Genetics; Justus-Liebig-University; Universities of Giessen and Marburg Lung Center, Member of the German Center for Lung Research; Giessen, Germany
Correspondence to: Reinhard H. Dammann, email: [email protected]
Keywords: AATK/ epigenetic regulation / DNA methylation/ human cancer/ tumor suppressor/ CTCF
Received: July 30, 2014
Accepted: August 18, 2014
Published: August 19, 2014
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ABSTRACT:
Epigenetic gene inactivation through promoter hypermethylation is an important aberration involved in the silencing of tumor-associated genes in cancer. Here we identified the apoptosis associated tyrosine kinase (AATK) as an epigenetically downregulated tumor related gene. We analyzed the epigenetic regulation of AATK in several human cancer cell lines and normal tissues by methylation and expression analysis. Hypermethylation of AATK was also analyzed in 25 primary lung tumors, 30 breast cancers and 24 matching breast tissues. In normal tissues the AATK CpG island promoter was unmethylated and AATK was expressed. Hypermethylation of AATK occurred frequently in 13 out of 14 (93%) human cancer cell lines. Methylation was reversed by 5-aza-2’-deoxycytidine treatment leading to re-expression of AATK in cancer cell lines. Aberrant methylation of AATK was also revealed in primary lung (40%) and breast (53%) cancers, but was found to be significantly less methylated in matching normal breast tissues (17%; p<0.01). In addition, we observed that AATK is epigenetically reactivated through the chromatin regulator CTCF. We further show that overexpression of Aatk significantly suppresses colony formation in cancer cell lines. Our findings suggest that the apoptosis associated tyrosine kinase is frequently inactivated in human cancers and acts as a tumor suppressive gene.
INTRODUCTION
Epigenetic modifications are important regulatory mechanisms for initiating and maintaining memory effects on gene expression. In mammals, these epigenetic mechanisms play essential roles in normal development through their effect on gene imprinting, X-chromosome inactivation and transcriptional inactivation of repetitive genomic elements. Moreover, epigenetic silencing of tumor suppressor genes is frequently observed in cancer [1]. In particular, de novo methylation of CpG island promoters is a hallmark of gene silencing during malignant transformation. CpG islands are sequences greater than 500 bp of GC-rich and CpG-dense elements in the genome. About 70% of known genes harbor a CpG island within -1 kb to +1 kb of their transcription initiation site. During tumorigenesis CpG island promoters become hypermethylated and this alteration is accompanied by the formation of a repressive chromatin and transcriptional silencing. Tumor suppressor genes that are frequently epigenetically inactivated are the Ras association domain family 1A (RASSF1A) gene and the cyclin-dependent kinase inhibitor 2A (p16) [1-3].
The CCCTC binding factor (CTCF) is a zinc finger-encoding protein involved in imprinting and chromosomal gene organization [4]. Several findings suggest that the CTCF insulator protein may contribute the boundaries at CpG island promoters [5-8]. Binding of CTCF is maintained in mitotic chromatin and may provide an epigenetic memory during cell division of proliferating cells [9]. Disruption of molecular boundaries mediated by CTCF may facilitate the epigenetic silencing of tumor suppressor genes [10, 11]. Recently, it has been shown that epigenetic downregulation of p16 and RASSF1A is associated with loss of CTCF binding and disappearance of a chromatin boundary [12].
The apoptosis associated tyrosine kinase (AATK) gene is localized on chromosome 17 at q25.3 [13]. Deletion of 17q25.3 was reported for several human cancers including oral, cervical and breast cancer [14-16]. The AATK protein that is also named AATYK or LMTK1 (lemur tyrosine kinase 1) consists of 1374 aa with the N-terminus harboring a protein tyrosine kinase domain (Fig. 1) [17]. AATK promotes neuronal differentiation and is induced during growth arrest and apoptosis of myeloid cells [18, 19]. Downregulation of AATK expression was reported for adenocarcinoma of the colon and for melanomas [20, 21]. In melanoma cells AATK overexpression inhibits growth and migration, and promotes apoptosis [21]. In our study we report frequent epigenetic inactivation of AATK in different human cancer entities (e.g. breast and lung) and its growth suppressive function in lung cancer. Furthermore, we show that the chromatin regulator CTCF induces epigenetic reactivation of AATK.
RESULTS
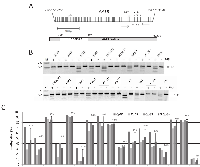
Methylation of AATK occurs in human cancers
We have performed a genome wide methylation screen in the lung cancer cell line H322 and have revealed a promoter specific hypermethylation of the apoptosis associated tyrosine kinase (AATK) gene (data not shown). The schematic promoter region of AATK and corresponding CpG islands is shown in Fig. 1A. The promoter lies within a CpG island of 527 bp on chromosome 17 from position 79’139’502 to 79’140’028 (UCSC genome browser). To reveal the epigenetic status of AATK in human cancers in more detail, we have analyzed the aberrant methylation of AATK in lung cancer (A549, A427, H322, H358, HTB-171), breast cancer (MCF-7, ZR75-1), melanoma (Sk-Mel13, IGR1), leiomyosarcoma (LMS6/93), follicular thyroid (FTC133), larynx cancer (HEP2), pancreas carcinoma cell line (PaCa2), cervix cancer (HeLa), HEK293 and human fibroblast (HF-55) by COBRA (Fig. 1B). Fragmentation of the PCR product indicates an underlying methylated AATK, whereas undigested PCR products originate from unmethylated AATK CpG island. In vitro methylated genomic DNA (i.v.m.) served as a methylated control (Fig. 1B). Normal human fibroblast (HF-55) and melanoma cells (Sk-Mel13) were unmethylated as analyzed by COBRA. COBRA data were confirmed by genomic bisulfite pyrosequencing of three CpGs within the AATK CpG island promoter (Fig. 1A and C). All lung cancer cell lines (A549, A427, H322, H358 and HTB171) were partially methylated for AATK (Fig. 1B and C). For breast cancer cell lines (MCF-7, ZR75-1), HeLa, LMS6/93, HEP2, Paca2, FTC133 and IGR1 partial methylation of AATK was also observed (Fig. 1B and C). Thus, a total of 15 cancer cell lines were analyzed, of which 14 (93%) were methylated for AATK. Therefore frequent hypermethylation of AATK was found in different human cancer entities, including lung and breast cancers.
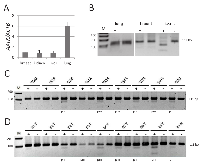
AATK hypermethylation in primary human breast and lung cancers
To analyze the impact of epigenetic regulation of AATK in carcinogenesis we investigated its expression and methylation in normal tissues as well as in breast and lung cancer samples (Fig. 2). Expression of AATK was found in normal breast, kidney and liver tissues and the highest expression was observed in normal lung tissues (Fig. 2A). AATK was unmethylated in normal lung and in three breast tissues isolated from healthy patients (Fig. 2B and data not show). Next we analyzed the aberrant methylation of AATK in 25 primary lung and 30 breast cancers by COBRA (Fig. 2). Four out of five lung adenocarcinomas (e.g. TA59) exhibited a partial methylation of AATK (Fig. 2C). However, aberrant AATK methylation was found in only two out of eight squamous lung tumors (e.g. TS37; Fig. 2C). In small cell lung cancer AATK was hypermethylated in four out of 12 tissues (data not shown). Thus, aberrant methylation of AATK was observed in 10 out of 25 (40%) of lung cancer samples. We also analyzed 30 primary breast tumors and 24 corresponding matching tissue controls (Figure 2D). 16 out of 30 (53%) breast cancer tissues were methylated, but AATK methylation was only found in 4 out of 24 (17%) matching control tissues (p<0.01, two tailed Fisher’s exact test). Moreover, methylation was weaker in the normal samples than in the corresponding tumor tissue (e.g. B9N and B9T, respectively; Fig. 2D). In summary, hypermethylation of AATK was also demonstrated in primary tumor tissues of cancer patients.
Decreased expression of AATK is associated with its hypermethylation in human cancer cell lines
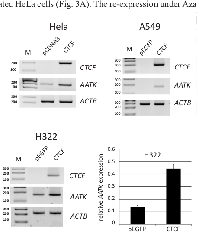
5-Aza-2’-deoxycytidine (Aza) inhibits DNA methylation [22] and is known to reverse hypermethylation of tumor suppressor genes, which can further lead to their re-expression [2]. We therefore chose HeLa, A549 and H322 with a methylated AATK CpG island for Aza treatment and analyzed AATK expression by RT-PCR as well as its methylation status (Fig. 3). The lung cancer cell line H322 has a methylated AATK promoter (Fig. 1 and 3) and shows very low endogenous AATK expression (Fig. 3A and B). RT-PCR shows that 5 µM Aza leads to AATK re-expression (Fig. 3A and B). Similar results are observed for the lung cancer cell line A459 and for HeLa cells, which are partially and strongly methylated respectively (Fig. 1 and 3). In HeLa and A549 the AATK expression is increased upon Aza treatment (Fig. 3A). FTC133 is partially methylated for the AATK promoter (Fig. 1B) and AATK expression is comparable to the Aza-treated HeLa cells (Fig. 3A). The re-expression under Aza treatment for HeLa, A549 and H322 was accompanied by AATK demethylation (Fig. 3C and D). H322 were 92% methylated and under Aza treatment methylation decreased significantly to 82% (5 µM Aza) (p=0.01, t-test; Fig. 3D). Genomic bisulfite pyrosequencing revealed a significant 10% demethylation at all three analyzed CpGs (p<0.05; Fig. 3D). In cancer cells, we observed an epigenetic silencing of the AATK CpG island promoter, which was reversed by Aza treatment as shown by AATK re-expression and promoter demethylation.
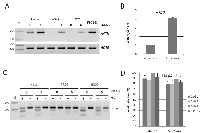
Regulation of AATK by the insulator protein CTCF
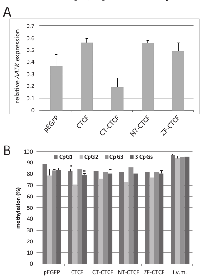
Previously, it has been reported that the CTCCC binding protein (CTCF) is involved in the epigenetic regulation of tumor suppressor genes [12]. Wendt and Barksi et al. have reported that downregulation of CTCF by RNA interference caused a twofold repression of AATK in HeLa cells [23, 24]. Database analysis of CTCF ChipSeq Encode data at the CTCFBSDB2.0 site revealed that CTCF binds the AATK CpG island in HUVEC. Furthermore, a search for potential CTCF-binding sites at the AATK locus found a match for a CTCF binding consensus site from position 79’139’590 to 79’139’599 (CCGCCAGGG) at CpG site 2 (Fig. 1A) (http://bsproteomics.essex.ac.uk: 8080/bioinformatics/ctcfbind.htm). To clarify whether CTCF could regulate AATK expression, we transfected CTCF in HeLa, A549 and H322 cancer cells (Fig. 4). Expression of CTCF induced the expression of AATK considerably in all three cell lines (Fig. 4). For H322 cells a 3.3 time increase in AATK expression was observed (Fig. 4). Since CTCF consist of three distinct domains: an N-terminal domain (NT), a zinc finger domain (ZF), which binds to DNA and a C-terminal (CT) domain. We generated deletion constructs of CTCF to investigate which of these domains is responsible for reactivation of AATK. Subsequently, we transfected these deletion constructs (CT-, NT- and ZF-CTCF), full length CTCF, and the empty EGFP vector control in H322 lung cancer cells (Fig. 5). Transfection of CTCF, NT-CTCF and ZF-CTCF induced the expression of AATK to levels similar to that of the control (Fig. 5A). Interestingly, a repression of AATK expression occurred for CT-CTCF transfection. To reveal if this induction is accompanied by changes in methylation levels we performed bisulfite pyrosequencing of three CpG sites (CpG1, CpG2 and CpG3) around the CTCF consensus site (Fig. 1A and Fig. 5B). Interestingly, we observed a slight and significant decrease from 84% to 79% average methylation of the three CpGs after control and CTCF transfection, respectively (p=0.03, t-test; Fig. 5B). This decrease was higher for CpG1 (89% to 83%; p=0.03) and CpG2 (79% to 71%, p=0.3), than for the third CpG (Fig. 5B). Similar demethylation was also observed after CT-CTCF, NT-CTCF and ZF-CTCF transfection (Fig. 5B). It is interesting to note that demethylation of the CpG site 2, which harbors the CTCF consensus site, was more pronounced after CTCF and NT-CTCF transfection compared to CT-CTCF and ZF-CTCF. In summary, we observed a reproducible demethylation of AATK after CTCF overexpression.
AATK expression reduces colony formation of cancer cells
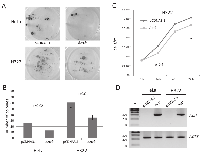
To functionally test AATK and its ability to suppress tumor formation, we performed colony formation and proliferation assays (Fig. 6). In order to do so we transfected HeLa and H322 with the Aatk expression- or empty control construct (pCDNA3.1) and selected with G418 for three weeks. Colonies were Giemsa stained and representative pictures are shown (Fig. 6A). In both cell lines AATK is methylated and downregulated (Fig. 1 and 3). After transfection of the Aatk containing construct, its expression was detected on RNA level (Fig. 6D). Expression of Aatk in these two cell lines significantly reduces the number of colonies (Fig. 6B). For both cell lines a twofold reduction in colonies was found (Fig. 6B). Moreover we analyzed proliferation of H322 cells after transient Aatk transfection. Cells were transfected with Aatk or empty vector (pCDNA3.1) and were counted over three days (Fig. 6D). After 24 hours a 25% reduction in cell number was found, however this reduction was not significant due to the observed variation (p=0.4). The significant reduction of colonies suggests that Aatk exhibits a tumor suppressive function in cancer cells.
DISCUSSION
In our study, we have identified the apoptosis associated tyrosine kinase (AATK) gene as a novel epigenetically inactivated target gene in human cancer. Hypermethylation of AATK was found in several epithelial cancer entities including lung, breast, skin, cervix, larynx and pancreatic cancer (Fig. 1 and Fig. 2). However, expression of AATK was found in normal tissues and its CpG island was unmethylated (Fig. 2). We observed that aberrant methylation of AATK is also frequently found in primary lung cancer (40%) and breast cancers (53%). Methylation frequency was significantly lower in matching normal tissues compared to breast cancers (17% compared to 53%, p<0.01). It is interesting to note that deletion of the chromosomal region which harbors the AATK gene is observed in different tumor entities including breast, cervix and oral cancers [14-16]. Since in general both alleles of a tumor suppressor gene are inactivated, these findings indicate that deletion and epigenetic inactivation are two important pathways for the inactivation of AATK in human cancer. It will be interesting to analyze if single nucleotide substitutions are present in the coding or regulatory sequences of AATK. Previously, downregulation of AATK at the protein level was only observed in colon polyps and melanoma, however the mechanism responsible for this downregulation was not revealed in detail [20, 21]. Thus, AATK hypermethylation may also be present in colon cancer and melanoma, as observed for the RASSF1A gene and other tumor suppressor genes [25-27]. Methylation of AATK has been reported in low grade serous ovarian neoplasms [28]. Here we report that AATK hypermethylation is frequently found in other human epithelial cancers, including primary breast and lung cancers (Fig. 2). Thus, it will be interesting to evaluate whether aberrant AATK methylation may represent a novel biomarker for prognostic or diagnostic purposes in human cancer.
By using 5-aza-2’-deoxycytidine treatment we were able to demethylate the AATK CpG island promoter and to re-express AATK (Fig. 3). This suggests that downregulation of AATK in cancer cell lines is due to its promoter hypermethylation. Moreover, we observed that expression of the epigenetic regulator CTCF also induced the expression and demethylation of AATK (Fig. 4 and Fig. 5). Previously, it has been reported that knockdown of CTCF by RNA interference caused twofold repression of AATK in HeLa cells [23, 24]. Here we show that overexpression of CTCF induced the expression of AATK in HeLa and in the lung cancer cell lines A549 and H322. It has been postulated that CTCF acts as a tumor suppressive factor [29, 30], since its function has been associated with altered expression of tumor-suppressor gene, such as E-cadherin, retinoblastostoma, RASSF1A or p16/CDKN2A [10, 12]. Disrupting the spectrum of target specificities of CTCF by mutations, its aberrant modifications (e.g. PARylation) or abnormal selective methylation of targets (e.g. loss of imprinting) could be associated with cancer [12, 31, 32]. Recurrent mutations of CTCF are mostly clustered in the conserved zinc finger domain [30]. It has been reported that CTCF-defective PARylation and dissociation from the molecular chaperone Nucleolin occur in p16-silenced cells, abrogating its function [12].Using CTCF mutants, the requirement of PARylation for optimal CTCF function has been demonstrated in transcriptional activation of the p19ARF promoter and inhibition of cell proliferation [33]. In our experiments we observed that full length CTCF and the N-terminal domain of CTCF, which harbor a intact PARylation site, induce the expression of AATK. In contrast, the C-terminal site of CTCF repressed AATK expression. CTCF has also been reported to act as a gene silencer [34]. Thus, the function of CTCF may require a specific composition of factors (e.g. PARP1) at CTCF binding sites. This has been suggested in a model where PARylation of CTCF is involved in gene activation [12]. Others have reported that SUMOylation of CTCF modulates a domain in CTCF that activates transcription and decondenses chromatin [35]. One SUMOylation site is also found in the N-terminal domain of CTCF. Thus, CTCF may act as a tumor relevant factor by inducing tumor relevant genes such as AATK. In the case of AATK this induction was also accompanied by a significant demethylation (Fig. 5B). Chipseq data reveal a CTCF binding site within the analyzed sequence. Thus, it will be interesting to analyze if increased CTCF binding occurs at its binding site upon CTCF expression. It has been shown that CTCF bound DNA remains unmethylated [36]. Stadler et al. showed that CTCF can bind to a pre-methylated CpG-poor target site, which in turn leads to localized demethylation [36]. However the exact mechanism of this local demethylation event has not been elucidated, and it could be passive by inhibiting the accessibility of binding sites for DNA methyltransferases during DNA replication. For this aspect, it is important to note that the demethylation caused by CTCF (5%) was half as much compared to Aza (10%), however Aza treatment took twice as long (4 days) compared to CTCF transfection (2 days). Additional chromatin changes (e.g. histone modification or structural alteration) could be involved in the CTCF-induced expression of AATK and should to be analyzed in further studies.
To verify the ability of AATK to suppress tumor growth as do other tumor suppressor genes, we performed colony formation and proliferation assays in human cancer cell lines (Fig. 6). Our results show that Aatk significantly suppresses colony growth in H322 lung cancer cells and HeLa cells, in which AATK is downregulated and inactivated by aberrant promoter methylation (Fig. 1 and Fig. 2). Recently, it has been reported that AATK inhibits cell proliferation, colony formation, migration, and also promotes apoptosis in melanoma cells [21]. Since we observed hypermethylation of AATK in IGR1 melanoma cell lines (Fig. 1), it will be interesting to analyze its methylation status in primary melanomas.
AATK was characterized as a novel kinase that induces and promotes neuronal differentiation in a human neuroblastoma cell line [19]. Thus it will be important to analyze if downregulation of AATK is associated with dedifferentiation of human epithelial cells. It has been reported that AATK interacts with the p35 activation subunit of the cyclin-dependent kinase 5 (CDK5) and is then phosphorylated by CDK5 [37, 38]. It has been suggested that AATK is a regulator of axonal outgrowth involving the RAB11 endosomal recycling pathway [17]. However, it is not known whether AATK phosphorylates any target proteins, and the function of AATK in epithelial cells has not been analyzed in detail.
In our study we demonstrate that AATK is frequently hypermethylated in human cancer cell lines and in primary lung and breast cancer samples. Demethylation of AATK is accompanied by re-expression of AATK in cancer cell lines. AATK expression was further found to be epigenetically regulated by CTCF. Additionally, ectopic expression of AATK suppresses colony formation. It will be fascinating to investigate the functional relationship of AATK epigenetic inactivation in cancer cell lines and their inability to differentiate or to undergo apoptosis. Therefore the loss of AATK might even promote dedifferentiation during tumorigenesis. Future research will elucidate the function of AATK during carcinogenesis.
MATERIALS AND METHODS
Tissue and cell lines
Primary cancer tissues and cancer cell lines were previously published: breast and lung [39-42]. All patients signed informed consent at initial clinical investigation. The study was approved by local ethic committees (City of Hope Medical Center, Duarte, USA and Martin-Luther University, Halle, Germany). All cell lines were cultured in a humidified atmosphere (37°C) with 5% CO2 and 1xPenicillin/Streptomycin in the recommended medium. Cells were transfected with 4 µg or 10 µg of constructs for 3.5 or 10 cm plates, respectively using Polyethylenimine or Turbofect (Fermentas GmbH, St.Leon-Rot, Germany).
Methylation analysis
DNA was isolated by phenol-chloroform extraction and then bisulfite treated prior to COBRA analysis and pyrosequencing [43]. 200 ng were subsequently used for PCR with primer AATKBSU1 (GGTTTGTATGGAAATTAATTTTTTTTT) and 5’-biotinylated primer AATKBSL2 (ATTTATACTAAAACCCAAAACCTACCC). Products were digested with 0.5 µl TaqI (Fermentas GmbH, St.Leon-Rot, Germany) 1 h at 65°C and resolved on 2% TBE gel. Methylation status was quantified utilizing the primer AATKSeq1 (GAGTTTAGTAGTAGAAGTAGT) and PyroMark Q24 (Qiagen, Hilden, Germany). Three CpGs are included in analyzed region of AATK and mean methylation was calculated. For in vitro methylation of genomic DNA we used M.SssI methylase (NEB, Frankfurt, Germany).
Expression analysis
RNA was isolated using the Isol-RNA lysis procedure (5 Prime, Hamburg, Germany). 25 µg of breast, kidney, liver and lung RNA of normal human samples (pools of five, one, three and four, respectively) were obtained from Agilent Technologies (Waldbronn, Germany). RNA was DNase (Fermentas GmbH, St.Leon-Rot, Germany) digested and then reversely transcribed [44]. RT-PCR was performed with primers: AATKRTF1: TGGCCTGGCTCACTGCAAGTACAG, AATKRTR1: CCCAGATGGTCACGCCCAGG, mAatkRTF1: GTGCTGAAGTGACCCCCTAC, mAatkRTR1: GGTCAGCGGTCACGAGATAG, ßACTFW: CCTTCCTTCCTGGGCATGGAGTC, ßACTRW: CGGAGTACTTGCGCTCAGGAGGA, GGCTCFRTFW: CAGGAAACGGAGGCTACGGTGG, GGCTCFRTRW: CCTCCTGCAGGCCTCCTTTGGA. Quantitative PCR (qRT-PCR) was performed in triplicate with PerfeCTa SYBR® Green (Quanta BioSciences, Gaithersburg, USA) using a Rotor-Gene 3000 (Corbett Research, Qiagen, Hilden, Germany).
Constructs
The cDNA of Aatk was obtained as a full length cDNA vector IRAVp968F06121D (Accession Number: BC080846; 5277 bp in pYX-Asc; imaGenes GmbH, Berlin, Germany) and cloned into the NotI and EcoRI sites of pcDNA3.1+. CTCF was a generous gift from Rainer Renkawitz (Justus Liebig University, Giessen, Germany) and deletion mutations were generated with QuickChange Lightning Site-Directed Mutagenesis Kit (Promega, Heidelberg, Germany).
List of Abbreviations
AATK: apoptosis associated tyrosine kinase; COBRA: combined bisulfite restriction analysis; Aza: 5-aza-2’-deoxycytidine, CTCF: CCCTC binding factor
Competing interests
The authors declare that they have no competing interests. The work was supported by grants (TRR81, LOEWE) from the DFG and Land Hessen to Reinhard Dammann. These organizations had no involvement in the study design, acquisition, analyses, data interpretation, writing of the manuscript and in the decision to submit the manuscript for publication.
Authors’ contribution
RHD has created the study. TH and RHD participated in the design of the study. TH, SKW, AMR and CH acquired data. TH, CH, SKW, AMR and RHD controlled, analyzed, and interpreted data. RHD prepared the manuscript. TH, CH, SKW, AMR and RHD read, corrected and approved the final manuscript.
ACKNOWLEDGEMENTS
The work was supported by grants (TRR81, LOEWE) from the DFG and Land Hessen to Reinhard Dammann. These organizations had no involvement in the study design, acquisition, analyses, data interpretation, writing of the manuscript and in the decision to submit the manuscript for publication. We would like to thank Rajkumar Savai for generous gift of materials and Helmut Dotzlaw for carefully reading the manuscript.
- 1. The epigenomics of cancer. Cell. 2007; 128(4):683-692. [PubMed] https://doi.org/10.1016/j.cell.2007.01.029.
- 2. Epigenetic inactivation of a RAS association domain family protein from the lung tumour suppressor locus 3p21.3. Nat Genet. 2000; 25(3):315-319. [PubMed]
- 3. The RASSF proteins in cancer; from epigenetic silencing to functional characterization. Biochim Biophys Acta. 2009; 1796(2):114-128. [PubMed]
- 4. CTCF: insights into insulator function during development. Development. 2012; 139(6):1045-1057. [PubMed]
- 5. DNA binding sites for putative methylation boundaries in the unmethylated region of the BRCA1 promoter. Int J Cancer. 2004; 111(5):669-678. [PubMed]
- 6. Putative zinc finger protein binding sites are over-represented in the boundaries of methylation-resistant CpG islands in the human genome. PLoS ONE. 2007; 2(11):e1184. [PubMed] https://doi.org/10.1371/journal.pone.0001184.
- 7. Boundaries between chromosomal domains of X inactivation and escape bind CTCF and lack CpG methylation during early development. Dev Cell. 2005; 8(1):31-42. [PubMed]
- 8. CTCF-dependent chromatin insulator is linked to epigenetic remodeling. Mol Cell. 2006; 23(5):733-742. [PubMed]
- 9. CTCF binding and higher order chromatin structure of the H19 locus are maintained in mitotic chromatin. Embo J. 2005; 24(18):3291-3300. [PubMed] https://doi.org/10.1038/sj.emboj.7600793.
- 10. Gain of DNA methylation is enhanced in the absence of CTCF at the human retinoblastoma gene promoter. BMC cancer. 2011; 11:232. [PubMed] https://doi.org/10.1186/1471-2407-11-232.
- 11. Epigenetic regulation of the human retinoblastoma tumor suppressor gene promoter by CTCF. Cancer Res. 2007; 67(6):2577-2585.
- 12. Epigenetic silencing of the p16(INK4a) tumor suppressor is associated with loss of CTCF binding and a chromatin boundary. Mol Cell. 2009; 34(3):271-284. [PubMed] https://doi.org/10.1016/j.molcel.2009.04.001.
- 13. Chromosomal assignment of a human apoptosis-associated tyrosine kinase gene on chromosome 17q25.3 by somatic hybrid analysis and fluorescence in situ hybridization. Journal of human genetics. 1999; 44(2):141142. [PubMed]
- 14. Detailed deletion mapping of chromosome 17q in ovarian and breast cancers: 2-cM region on 17q21.3 often and commonly deleted in tumors. Cancer Res. 1993; 53(14):3382-3385. [PubMed]
- 15. Genomic instability and tumorspecific alterations in oral squamous cell carcinomas assessed by inter-(simple sequence repeat) PCR. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003; 9(3):1057-1062. [PubMed]
- 16. Three distinct regions of allelic deletion on chromosome. . 2003; 9(3):1057-1062. [PubMed]
- 17 involved in sporadic gastric cancer. 2008; 55(85):1487-1491.17. Takano T, Tomomura M, Yoshioka N, Tsutsumi K, Terasawa Y, Saito T, Kawano H, Kamiguchi H, Fukuda M and Hisanaga S. LMTK1/AATYK1 is a novel regulator of axonal outgrowth that acts via Rab11 in a Cdk5-dependent manner. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012; 32(19):6587-6599.
- 18. AATYK: a novel tyrosine kinase induced during growth arrest and apoptosis of myeloid cells. Oncogene. 1997; 15(25):31273135. [PubMed]
- 19. A novel kinase, AATYK induces and promotes neuronal differentiation in a human neuroblastoma (SH-SY5Y) cell line. Brain research Molecular brain research. 2000; 77(2):151-162. [PubMed]
- 20. Differential expression in normal-adenoma-carcinoma sequence suggests complex molecular carcinogenesis in colon. Oncology reports. 2006; 16(4):747-754. [PubMed]
- 21. Apoptosis-associated tyrosine kinase 1 inhibits growth and migration and promotes apoptosis in melanoma. Laboratory investigation; a journal of technical methods and pathology. 2014; 94(4):430-438. [PubMed]
- 22. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980; 20(1):85-93. [PubMed]
- 23. High-resolution profiling of histone methylations in the human genome. Cell. 2007; 129(4):823-837. [PubMed]
- 24. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature. 2008; 451(7180):796-801.
- 25. Hypermethylation of the CpG island of the RASSF1A gene in ovarian and renal cell carcinomas. Int J Cancer. 2001; 94(2):212-217. [PubMed]
- 26. Frequent intratumoural heterogeneity of promoter hypermethylation in malignant melanoma. Histol Histopathol. 2007; 22(9):10051015. [PubMed]
- 27. RASSF10 promoter hypermethylation is frequent in malignant melanoma of the skin but uncommon in nevus cell nevi. J Invest Dermatol. 2012; 132(3 Pt 1):687-694. [PubMed]
- 28. Distinct DNA methylation profiles in ovarian serous neoplasms and their implications in ovarian carcinogenesis. American journal of obstetrics and gynecology. 2010; 203(6):584 e581-522. [PubMed] https://doi.org/10.1016/j.ajog.2010.08.003.
- 29. The tumor suppressor role of CTCF. Journal of cellular physiology. 2012; 227(2):479492. [PubMed]
- 30. The cancertestis antigen BORIS phenocopies the tumor suppressor CTCF in normal and neoplastic cells. Int J Cancer. 2013; 133(7):1603-1613. [PubMed]
- 31. Frequent aberrant methylation of the imprinted IGF2/H19 locus and LINE1 hypomethylation in ovarian carcinoma. International journal of oncology. 2010; 36(1):171-179. [PubMed]
- 32. CTCF is a uniquely versatile transcription regulator linked to epigenetics and disease. Trends in genetics : TIG. 2001; 17(9):520-527. [PubMed]
- 33. Mutational analysis of the poly(ADP-ribosyl)ation sites of the transcription factor CTCF provides an insight into the mechanism of its regulation by poly(ADP-ribosyl)ation. Mol Cell Biol. 2010; 30(5):1199-1216. [PubMed] https://doi.org/10.1128/MCB.00827-09.
- 34. The insulator protein CTCF represses transcription on binding to the (gt)(22)(ga)(15) microsatellite in intron 2 of the HLA-DRB1(*)0401 gene. Gene. 2000; 253(2):209-214. [PubMed]
- 35. Sumoylation modulates a domain in CTCF that activates transcription and decondenses chromatin. Journal of cellular biochemistry. 2010; 111(3):665-675. [PubMed]
- 36. DNA-binding factors shape the mouse methylome at distal regulatory regions. Nature. 2011; 480(7378):490-495. [PubMed]
- 37. Apoptosis-associated tyrosine kinase is a Cdk5 activator p35 binding protein. Biochem Biophys Res Commun. 2003; 310(2):398-404. [PubMed]
- 38. Phosphorylation of AATYK1 by Cdk5 suppresses its tyrosine phosphorylation. PLoS One. 2010; 5(4):e10260. [PubMed] https://doi.org/10.1371/journal.pone.0010260.
- 39. Frequent epigenetic inactivation of cystatin M in breast carcinoma. Oncogene. 2007; 26(21):3089-3094. [PubMed]
- 40. CpG island methylation and expression of tumour-associated genes in lung carcinoma. Eur J Cancer. 2005; 41(8):1223-1236. [PubMed]
- 41. Hypermethylation of the cpG island of Ras association domain family 1A (RASSF1A), a putative tumor suppressor gene from the 3p21.3 locus, occurs in a large percentage of human breast cancers. Cancer Res. 2001; 61(7):3105-3109. [PubMed]
- 42. Frequent hypermethylation of RASSF1A tumour suppressor gene promoter and presence of Merkel cell polyomavirus in small cell lung cancer. Eur J Cancer. 2009; 45(12):2207-2211. [PubMed]
- 43. Increased DNA methylation of neuropsychiatric genes occurs in borderline personality disorder. Epigenetics : official journal of the DNA Methylation Society. 2011; 6(12):1454-1462.
- 44. The tumor suppressor RASSF10 is upregulated upon contact inhibition and frequently epigenetically silenced in cancer. Oncogenesis. 2012; 1:e18. [PubMed] https://doi.org/10.1038/oncsis.2012.18.
Last Modified: 2016-06-21 20:31:05 EDT
PII: 28
