Genes & Cancer
Taurolidine cooperates with antineoplastic drugs in neuroblastoma cells
Georg Eschenburg1, Christian Luckert1, Konrad Reinshagen1 and Robert Bergholz1
1 Department of Pediatric Surgery, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Correspondence to: Georg Eschenburg, email: [email protected]
Keywords: Neuroblastoma, Apoptosis, Vincristine, Doxorubicin, Experimental Therapies
Received: September 29, 2014
Accepted: October 08, 2014
Published: October 09, 2014
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ABSTRACT
Neuroblastoma is the most common extracranial tumor in childhood. Outcome of stage 4 disease remains poor and the development of novel therapeutic approaches is thus urgently needed. Taurolidine (TRD), originally invented to avoid catheter infections, has shown to exhibit antineoplastic activity in various cancers. The growth of neuroblastoma cell lines is inhibited by TRD as recently demonstrated. Further analysis disclosed a significant negative growth effect of TRD on the four neuroblastoma cell lines SH-EP TET21N, SK-N-AS, SK-N-BE(2)-M17 and SK-N-SH. Detected IC50 (51-274 µM; 48 h) are promising and correspond to clinically- achievable plasma levels. Apoptosis was induced (76-86%; 48 h) in a time- dependent manner mediated by a simultaneous activation of the intrinsic and extrinsic pathways. This was confirmed by cleavage of caspases -3, -8 and -9 and abrogation of apoptosis by pan-caspase inhibition. Application of TRD resulted in a significant enhancement of cytotoxic drugs vincristine/doxorubicin (2/3 of 4 cell lines) making TRD a promising candidate to be included in neuroblastoma therapy regimens in the future.
INTRODUCTION
Neuroblastoma, originating from sympathetic nervous tissue, is the most common extracranial tumor during childhood. Due to significant improvements in therapy in the recent years 5-year survival rates for non-high risk disease are good (>90%) [1]. However, stage 4 disease with distant organ metastases is highly common at diagnosis.
At the same time, outcome remains poor despite primary surgery and chemotherapy as well as intensive treatment protocols including megatherapy followed by blood stem cell transplantation, differentiation therapy, or immunotherapy [2].
Therefore, the development of novel therapeutic approaches for the treatment of neuroblastoma is one of the main objectives in pediatric oncology [3, 4].
The drug taurolidine [bis(1,1-dioxoperhydro-1,2,4-thiadiazinyl-4)methane] (TRD) is derived from the aminosulfone acid taurine that is able to protect mammalian tissue from oxidant-induced injury [5]. It was originally synthesized by Geistlich-Pharma. TRD was initially used for its antiinflammatory and antimicrobial properties in the treatment of surgical and wound infections as well as for preventing infections by central venous catheters [6-8].
In recent years, substantial evidence was presented for antineoplastic activities of TRD. Cell growth inhibition and induction of apoptosis was achieved in vitro and in vivo using animal models in such different tumors as bladder carcinoma [9], colon cancer [10-12], epithelioid cell sarcoma [13], esophageal cancer [14], fibrosarcoma [10, 15], gallbladder cancer [16], glioblastoma [17, 18], leiomyosarcoma [13], lung cancer [19], mesothelioma [20], melanoma [21, 22], osteosarcoma [23], ovarian cancer [19], pancreas carcinoma [10], prostate cancer [24] and rhabdomyosarcoma [13].
In neuroblastoma TRD significantly inhibited the cell growth of cell lines SK-N-BE(2)-M17 and SK-N-SH [25]. However, the underlying mechanisms of TRDs mode of action in neuroblastoma cells are unknown so far.
In the extrinsic pathway of apoptosis binding of specific ligands like TNF-α, TRAIL or FASL to so called death receptors on the cell surface initiates the cytosolic formation of complex II containing caspase-8 leading to its activation and caspase-3 subsequently [26].
The intrinsic apoptosis pathway is activated by a plethora of stimuli like viral infections, radiation or treatment with cytotoxic drugs used in chemotherapy [27]. In consequence, processes in the mitochondria evoke a decrease in the mitochondrial membrane potential (MMP), a release of cytochrome C and formation of a complex called apoptosome containing apoptotic protease activating factor 1 (Apaf-1) and caspase-9. The latter is cleaved and in turn activates the effector caspase-3 thereby initiating processes cumulating in apoptosis.
Promising results were obtained with TRD in patients as well. A comprehensive clinical evaluation of TRD is nevertheless missing so far. In one patient with gastric cancer histological remission of the tumor growth was reached. The patient however died from myocardial infarction after occurrence of primary urothelial carcinoma [28]. In stage IV melanoma patients TRD enhanced the tolerability of high-dose interleukin 2 [29].
In two glioblastoma patients TRD was able to significantly improve the quality of life with partial remission of tumor burden [30]. No relevant toxicity was detectable in patients therefore making TRD a promising candidate for further research [28, 31].
Mechanistic analysis of TRD-mediated cell death in cancer cells revealed apoptosis as the main mode of action. Dependent on cell type and experimental setup decrease in mitochondrial membrane potential, cytochrome C release and cleavage of caspase-9 was detected characteristic for the activation of the intrinsic pathway [14, 22, 24, 32, 33]. An activation of the death receptor initiated extrinsic pathway with activation of caspase-8 was observed as well [17, 22, 33].
In the current study we, for the first time, present detailed evidence that TRD significantly induces apoptosis via activating the intrinsic and extrinsic pathways in different neuroblastoma cell lines. TRD cooperates with the cytotoxic drugs doxorubicin and vincristine that are currently used in therapy regimens for neuroblastoma making TRD a promising novel candidate for treatment of this malicious and defying childhood disease.
RESULTS
Inhibition of Cellular Growth by Taurolidine in Neuroblastoma Cell Lines
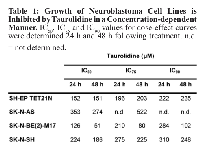
We first evaluated the growth of human neuroblastoma cell lines (n=4) in the presence of increasing amounts (0-500 µM) of taurolidine (TRD). Cellular growth was inhibited by TRD in all cell lines. Decrease in growth 24 h following treatment initiation was seen with a minimal dose of 100 µM TRD (figure 1A). The cell line SK-N-BE(2)-M17 was most sensitive with an IC50 of 126 µM, the IC50 of the other cell lines varied between 152-353 µM (table 1).
Analysis of cellular growth after 48 h of treatment revealed a stronger impact of TRD in comparison to the 24 h time point as expected (figure 1B). IC50 values were between 51 µM (SK-N-BE(2)-M17) and 274 µM (SK-N-AS) at 48 h (table 1).
Neuroblastoma Cells Undergo TRD-induced Apoptosis
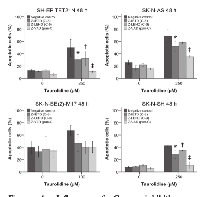
In order to evaluate if apoptosis is causal for the TRD-induced decrease of cell growth neuroblastoma cells were analyzed using flow cytometry.
In all cell lines TRD induced apoptosis characterized by externalization of phosphatidylserine (Annexin V staining) and PI uptake (figures 2A-B). Apoptotic cells significantly increased (p ≤ 0.05) in a time- and concentration-dependent way with a maximum apoptosis of 86% in the cell line SH-EP TET21N (500 µM TRD; 48 h) (figure 2B).
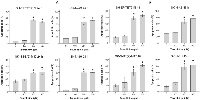
To generate additional evidence for TRD’s potential to stimulate the induction of apoptosis in neuroblastoma the cleavage of caspase-3 to its active fragment, a specific feature of apoptosis, was determined [34].
Treatment with TRD caused a significant induction of active caspase-3 in a concentration-dependent manner (figure 3). Cleavage of caspase-3 was significantly inhibited (p ≤ 0.05) in three of four cell lines if cells were additionally pre-treated with pan-caspase inhibitor Z-VAD (figure 3) giving evidence that the impact of TRD on apoptosis is mediated by upstream caspases activation.
Taurolidine-mediated Apoptosis is Abolished by Pan-Caspase-inhibition
For further characterization of the molecular pathways involved in TRD-induced apoptosis specific and pan-caspase inhibition was conducted.
Neuroblastoma cells were treated with TRD for 48 h and apoptosis was quantified by flow cytometry. TRD-induced apoptosis was reduced by inhibition of caspase-8 or caspase-9 in all four neuroblastoma cell lines respectively (figure 4).
The apoptosis reduction was significant (p ≤ 0.05) using caspase-8 inhibitor in three of four cell lines and with caspase-9 inhibitor in two of four cell lines.
Consistent with the former findings pan-caspase inhibition, by irreversibly binding the active site of activated proteases using Z-VAD, significantly (p ≤ 0.05) reduced the effect of TRD to basal levels in three of four cell lines (figures 3 and 4).
Taurolidine Induces Activation of Intrinsic and Extrinsic Apoptosis Pathways
After relevant inhibition of TRD-mediated apoptosis was achieved by blockade of active caspases-8 and -9 the effects of TRD on the activation of the in- and extrinsic apoptosis pathways was quantified by flow cytometry.
Treatment with TRD significantly induced (p ≤ 0.05) the formation of active caspase-8 and -9 in a concentration-dependent way indicative for extrinsic and intrinsic apoptosis in the cell lines SK-N-AS and SK-N-BE(2)-M17 (figures 5A-B). The activation of both caspases was significantly impeded with Z-VAD pan-caspase inhibitor treatment in all cell lines with the exception of SH-EP TET21N (figures 5A-B).
Apoptosis Induction of TRD in Combination with Vincristine and Doxorubicin
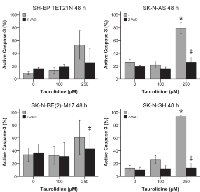
The vinca alkaloid vincristine and the antracycline doxorubicin are antineoplastic drugs commonly used for neuroblastoma chemotherapy [3]. Thus, it was tested if TRD has the potential to improve the effects of these drugs.
All cell lines were susceptible for 25 nM vincristine with an induction of apoptosis of 35-57% after 48 h (figure 6A). The combination of vincristine with TRD resulted in an apoptosis induction of 35-79% and a significant increase (p ≤ 0.05) of the vincristine effect in the cell lines SH-EP TET21N and SK-N-BE(2)-M17 (figure 6A).
Treatment of neuroblastoma cell lines with 250-500 nM doxorubicin effected an apoptosis induction between
Last Modified: 2016-10-03 14:56:01 EDT
