Genes & Cancer
Histone acetyltransferases and histone deacetylases in B- and T-cell development, physiology and malignancy
Leila Haery1, Ryan C. Thompson1 and Thomas D. Gilmore1
1 Department of Biology, Boston University, Boston, MA, USA
Correspondence to: Thomas D. Gilmore, email: [email protected]
Keywords: HAT, HDAC, acetylation, B cells, T cells
Received: May 1, 2015
Accepted: May 12, 2015
Published: May 15, 2015
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ABSTRACT
The development of B and T cells from hematopoietic precursors and the regulation of the functions of these immune cells are complex processes that involve highly regulated signaling pathways and transcriptional control. The signaling pathways and gene expression patterns that give rise to these developmental processes are coordinated, in part, by two opposing classes of broad-based enzymatic regulators: histone acetyltransferases (HATs) and histone deacetylases (HDACs). HATs and HDACs can modulate gene transcription by altering histone acetylation to modify chromatin structure, and by regulating the activity of non-histone substrates, including an array of immune-cell transcription factors. In addition to their role in normal B and T cells, dysregulation of HAT and HDAC activity is associated with a variety of B- and T-cell malignancies. In this review, we describe the roles of HATs and HDACs in normal B- and T-cell physiology, describe mutations and dysregulation of HATs and HDACs that are implicated lymphoma and leukemia, and discuss HAT and HDAC inhibitors that have been explored as treatment options for leukemias and lymphomas.
INTRODUCTION
B and T cells have a variety of cellular subtypes that arise through a complex series of developmental events. The function of these various immune cell subtypes can be altered by numerous extracellular factors, including antigens, cytokines, and growth factors. Many of these developmental and functional processes are controlled by large-scale changes in gene expression, either due to epigenetic changes in chromatin structure or to the activity of specific transcription factors (TFs). Histone acetyltransferases (HATs) and histone deacetylases (HDACs) are two opposing classes of enzymes that play widespread roles in regulating transcription either by altering chromatin structure or by modulating the activity of specific TFs. Thus, it is perhaps not surprising that HATs and HDACs play roles in maintaining hematopoietic precursors and in coordinating their maturation into various subtypes of B and T cells.
As with many proteins that have important roles in normal developmental and cell-specific proliferation and survival processes, HAT and HDAC activity is altered in many B- and T-cell malignancies. Moreover, several HDAC inhibitors (HDACi) have been found to reduce the proliferation of B and T cancer cells in vitro and in vivo. As an outcome of such basic research, there are four FDA-approved HDACi being used clinically to treat T-cell lymphoma and multiple myeloma, and there are several clinical trials using HDACi for the treatment of B- and T-cell cancers.
In this review, we describe the roles of HATs and HDACs in normal B- and T-cell development and function, and also discuss alterations in HAT/HDAC activity in B- and T-cell malignancies. Finally, we summarize the current status of HAT and HDAC inhibitors as potential therapies for cancers affecting B and T cells.
Overview of the Regulation of Transcription by HATs and HDACs
HATs and HDACs carry out acetylation and deacetylation, respectively, of the ε-amino group of specific lysine residues on target proteins. The addition of an acetyl group prevents the formation of positive charges on the lysine amino group, and thus, can affect protein activity. Through this reversible catalytic event, HATs and HDACs can regulate transcription in two general ways: 1) by altering histone acetylation patterns, thereby modulating chromatin structure and its accessibility to transcriptional regulatory proteins [1, 2], and 2) by acetylating and affecting the activity of non-histone substrates that directly regulate transcription, including a diverse array of TFs [3].
HATs are a subtype of transcriptional coactivators, in that they possess intrinsic acetyltransferase activity and can enhance the ability of a TF to activate transcription. In general, HAT-mediated acetylation of nucleosomal histones increases the accessibility of DNA to TFs and leads to increased transcription at a given DNA locus. Acetylation of specific TFs by HATs can also increase their ability to bind DNA, resist proteasomal degradation, or interact with other TFs or coactivators, and consequently, direct acetylation of TFs can also be a transcriptional activating event [3]. In addition, by serving as protein scaffolds, HATs can promote the formation of transcriptional activating complexes near a gene promoter. This scaffolding function does not necessarily require HAT enzymatic activity, but rather is defined by the protein-interaction domains of these relatively large molecules.
HDACs, on the other hand, generally act as transcriptional corepressors by deacetylating nucleosomal histones, which can lead to chromosomal condensation and the exclusion of transcriptional activating complexes. Additionally, large HDAC-containing repressor complexes can localize to specific gene loci and exclude activating molecules, including HATs, from interacting with TFs. HDACs can also deacetylate specific TFs, decreasing their DNA-binding activity, inducing their degradation, or changing their subcellular localization or protein-protein interactions [4].
Families of Human HATs and HDACs HAT families
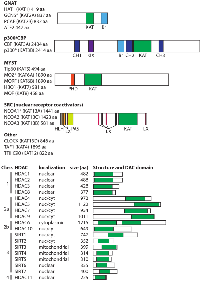
There are 17 human HATs, which are divided into five families based primarily on the extent of sequence similarity [5] (Figure 1). Although HATs can act on a broad range of substrates in vitro, HATs are usually directed to specific targets in vivo, and thus, HAT families generally have distinct biological functions. The non-catalytic domains of HATs are responsible for dictating this substrate specificity, and HAT families generally have conserved protein-protein interaction and reader domains (e.g., bromodomains, PHD fingers), which enable them to localize to particular genomic sites and recognize specific chemical or epigenetic modifications. The size of the catalytic HAT domain and the mechanism of catalysis also differ between HAT families. For example, CBP and p300 utilize a “hit and run” kinetic model defined by an initial binding of acetyl-CoA followed by transient binding to the target lysine [6, 7], whereas the GNAT family HATs adopt a ternary complex during catalysis [8].
GNAT family HATs (GCN5, HAT1, PCAF, ATF2) are generally part of large, multi-protein complexes that contain TBP-associated factors (TAFs) and a single catalytic HAT subunit (reviewed in [9]). Two well-characterized complexes found in humans are the 700 kDa (i.e., ATAC) and 2 MDa (i.e., TFTC, STAGA, and PCAF) complexes. These large HAT-containing complexes play roles in global chromatin acetylation (i.e., the deposition of acetyl marks on histones) and as coactivators of genes when recruited to DNA by specific TFs or regulatory proteins. Members of the GNAT family, especially PCAF, also acetylate specific TFs and modulate their activity (e.g., p53, BRCA2, PTEN). GNAT family members have a conserved C-terminal bromodomain, which has been shown to be an acetyl-lysine targeting motif. GNAT family member ATF2 is the only sequence-specific DNA-binding transcriptional activator with intrinsic HAT activity.
The CBP/p300 HATs are large (~300 kDa), highly related proteins with a single HAT domain, a bromodomain, and several cysteine–histidine-rich (CH) domains that participate in a variety of protein-protein interactions [5]. Indeed, CBP/p300 have been shown to acetylate over 75 target proteins, including all histone proteins, as well as numerous TFs. By virtue of their multiple protein-protein interaction domains, CBP/p300 can also promote transcriptional activation by nucleating transcriptional complexes at promoters in a non-enzymatic manner. Although they generally act as coactivators, in some cases, CBP/p300 appear to be involved in transcriptional repression [10].
The MYST family of HATs (TIP60, MOZ, MORF, HBO1, MOF) is characterized by a conserved MYST domain that contains the catalytically active HAT domain. The two largest family members, MOZ and MORF, also have a PHD zinc finger domain, which recognizes methyl-lysine-containing motifs [11], and a C-terminal transactivation domain that interacts with various transcription factors, including hematopoietic cell regulators PU.1 and Runx1 [12-14]. Most MYST family HATs act as catalytic subunits of large multiprotein complexes, including the ING family of tumor suppressors [15]. The steroid receptor coactivators (SRCs) include three HATs (NCOA1, NCOA2, NCOA3) that enhance transcription of genes responsive to liganded nuclear receptors [16]. In addition to the HAT domain, SRCs contain three conserved domains: 1) an N-terminal bHLH-PAS (basic helix-loop-helix-Per/ARNT/Sim), which is necessary for interaction with other coactivators; 2) one or more LXXL repeats, which mediate interactions with other nuclear receptors and cofactors; and 3) two C-terminal transcriptional activation domains (AD1 and AD2). Although SRCs have been associated with various human cancers, currently they are not known to have a role in hematopoiesis or B-/T-cell function.
Other HATs that are not clearly part of a family include the following; TAF1 (TAFII 250), a subunit of the TFIID general TF complex; CLOCK, which is primarily involved in circadian rhythm; and the 90 kDa subunit of TFIIIC, which is involved in the control of general transcription in a complex with RNA polymerase III.
HDAC families
To counterbalance the impact of HATs on protein function and genome structure, there are 18 human HDACs, which are commonly divided into four major classes based on homology to yeast orthologs (Figure 1): class 1 (HDAC1, 2, 3 and 8), class 2 (HDAC4, 5, 6, 7, 9, and 10), class 3 (aka sirtuins; SIRT1, 2, 3, 4, 5, 6, and 7), and class 4 (HDAC11) [17-20]. HDAC classes differ in their structure, substrate specificity, enzymatic mechanism, subcellular localization, and tissue-specific expression. Even though most HDACs contain a nuclear localization signal (NLS) and, in some cases, a nuclear export signal (NES), HDACs often localize to specific subcellular regions due to protein-protein interactions with proteins that direct their cellular localization.
The “classical” HDACs are those in classes 1, 2a, 2b, and 4, and they have a conserved ~390 aa catalytic domain and Zn2+-dependent deacetylase activity. The conserved, ~275 aa catalytic domain of the class 3 sirtuins is NAD+-dependent and unrelated to the catalytic domain of the classical HDACs [21, 22]. These differences in their catalytic mechanisms have implications for inhibition of HDAC activity, and thus, many of the HDAC inhibitors (HDACi) used in cancer therapeutics target the classical HDACs (discussed below).
Class 1 HDACs are ubiquitously expressed and localize almost exclusively to the nucleus. The class 2 HDACs are generally much larger than class 1 HDACs, show tissue-specific expression patterns, and often shuttle in and out of the nucleus. In general, HDACs in both classes 1 and 2 are found in large transcriptional repressing complexes, and are recruited to DNA either by other proteins in those complexes or by other DNA-binding proteins. These large protein complexes play roles in HDAC localization and substrate specificity, can act as scaffolds to recruit DNA-binding proteins, and provide the cofactors required for HDAC function. Indeed, lack of these cofactors limits the activity of some recombinant HDACs [23]. HDAC 11 is the only class 4 HDAC, and although it shows sequence similarity to class 1 and 2 HDACs, it does not exist within any of the known HDAC complexes.
Class 3 sirtuins vary in their subcellular localization and interact with a diverse array of TFs and other, primarily non-histone, substrates [24]. For example, SIRT1 can directly interact with substrates involved in the stress response, including p53, FOXO proteins, and NF-κB. The mitochondrially-localized sirtuins (SIRT3, 4, 5) can regulate mitochondrial function, respiration, and energy consumption [25]. Some sirtuins (SIRT4 and 6) lack deacetylase activity, but possess ADP-ribosyl-transferase activity and play roles in metabolism and DNA repair.
HATs and HDACs in B- and T-cell Development HATs and HDACs in early hematopoietic development
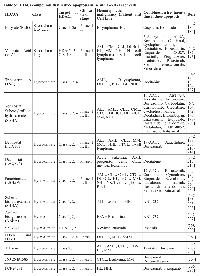
B- and T-cell development involves controlled stages of gene expression programs and genomic instability, which ultimately give rise to the diversity of cells that provide adaptive immunity. These developmental stages are tightly regulated by a large variety of TFs and are coupled with the accessibility of DNA to factors that coordinate chromosomal rearrangements. HATs and HDACs play major roles in normal B- and T-cell development because they can interact with hematopoietic regulators and TFs, as well as affect DNA accessibility by modifying chromatin structure near relevant target genes. In this section, some roles of HATs and HDACs in normal hematopoiesis, lymphopoiesis, and B- and T-cell function are discussed (summarized in Table 1).
Much of what is known about the role of HATs and HDACs in the development of mammalian B and T cells comes from the study of whole knockout (KO) mice and of mice with tissue-specific inactivation of individual HATs and HDACs. In most cases, whole-mouse HAT and HDAC KOs are embryonic lethal. Therefore, to explore the roles of these HATs/HDACs in hematopoiesis, either hematopoietic progenitors have been isolated from KO mice or hematopoietic lineage-specific gene KOs have been generated.
Mice with cell-specific KOs of CBP or p300 have defects in maintenance and differentiation of hematopoietic stems cells (HSCs) [26, 27]. The defect in hematopoiesis in p300-null stem cell lines can be rescued by re-expression of wild-type p300 or when an extra copy of CBP is placed under control of the p300 locus, suggesting that the total dosage of HAT activity by CBP/p300 is critical for hematopoietic maintenance and differentiation rather than the specific activity of either individual HAT [26, 27]. Likewise, in the MYST family, whole animal KO of MOZ is embryonic or perinatal lethal, and MOZ KO embryos show a dramatic reduction in the number and repopulation capacity of hematopoietic progenitors, whereas mice with heterozygous KO or with a HAT deletion of MOZ often show intermediate phenotypes, suggesting a dose-dependent requirement for activity of this HAT [13, 28].
To study the role of specific p300 domains in hematopoiesis, a series of p300 deletion mutants were re-expressed in p300-null embryonic stem cells (ESCs), and their ability to contribute to hematopoiesis was analyzed. These studies showed that p300 mutants lacking the KIX or CH1 domain had reduced abilities to induce hematopoiesis, and these defects were similar to the parental p300-null cells [27]. This reduction in hematopoiesis is thought to be due to an inability of KIX and CH1 deletion mutants to interact with the TF MYB [27, 29]. Interestingly, the presence of a functional HAT domain in p300 appears to play a role in limiting the proliferation of hematopoietic precursors, in that expression of a HAT-deficient p300 mutant in p300-null cells leads to increased numbers of hematopoietic cell populations, as compared to re-expression of wild-type p300 [27]. The dispensability of the CBP/p300 HAT domain for hematopoiesis may be due to their interaction with the HAT PCAF, which provides catalytic HAT activity in some CBP/p300 signaling contexts (e.g., myogenic differentiation) [30]. Thus, unlike the MYST family, which relies on HAT activity for proper proliferation of HSCs (described below), the HAT domains of CBP/p300 may actually reduce the proliferation of some hematopoietic cell types.
Among the MYST family proteins, the role of MOZ in hematopoiesis was determined by analyzing the hematopoietic progenitors in MOZ KO mice. Whole-mouse MOZ KO reduces the number of HSCs, and also affects the ability of these stem cells to renew and reconstitute the hematopoietic system [13]. HSCs from MOZ KO mice have reduced HOXA9 expression, which is known to reduce the differentiation potential of HSCs. MOZ is also a transcriptional coactivator of PU.1, and reduced PU.1 activity can explain many of the phenotypes seen in MOZ KO mice [13, 31, 32]. In mice expressing HAT-deficient MOZ, hematopoietic progenitors are severely defective in competitive repopulation assays, demonstrating a critical role of the MOZ catalytic domain in HSC functionality [33]. These defects were linked to a marked deficiency in the proliferative capacity of HAT-deficient MOZ precursors [33]. Loss of MYST family member MOF in ESCs is associated with a reduction in the expression of some hematopoietic genes, suggesting a role for MOF in hematopoiesis [34]. Additionally, conditional KO of the coactivator TRRAP, which can act as a subunit of TIP60 and PCAF HAT-containing complexes, leads to loss of HSCs [35].
HDAC1 and 2 have overlapping critical roles in early hematopoiesis and HSC homeostasis, largely by acting in a SIN3A/HDAC1/2-repressor complex. While mice with bone marrow-specific deletions of either HDAC1 or HDAC2 show only moderate phenotypes, the simultaneous deletion of HDAC1 and HDAC2 (or SIN3A alone) leads to nearly complete loss of hematopoietic progenitors, causing severe reduction in the numbers of spleen, thymic and bone marrow cells [36-38]. During HSC emergence in zebrafish, HDAC1 is recruited to the erk promoter by SMAD1/5, and represses erk1/2 expression by deacetylating H3K9 and H3K27 [39]. Conditional KO studies have shown that HDAC3 is required for DNA replication in HSCs, which is essential for their ability to produce B- and T-cell progenitors [40].
HATs and HDACs in B-cell development and function
Disruption of p300 or CBP at the pro-B cell stage results in a 25-50% reduction in the number of B cells in the peripheral blood; however, the number of pro-B, pre-B, and immature B cells in the bone marrow is unaffected [41]. Loss of CBP at this stage does not drastically perturb gene expression in resting B cells, as ~99% of microarray transcripts measured in CBP-null cells were within 1.7-fold of controls [41]. These results indicate that loss of either p300 or CBP starting at the pro-B cell stage is not required for B-cell function, possibly due to functional redundancy of these two HATs. In contrast to the single KOs, the double KO of CBP and p300 in pro-B cells causes a dramatic reduction in the number of peripheral B cells [41].
With the exception of mature B cells, the HAT activity of MOZ is required for the cell proliferation required to maintain healthy numbers of hematopoietic precursors.That is, mice expressing a HAT-deficient MOZ protein show an approximately 50% reduction in the numbers of pro/pre-B cells and immature B cells, whereas the number of mature B cells and their ability to carry out antibody responses is unaffected [33].
KO of GCN5 in the chicken immature B-cell line DT40 showed that GCN5 regulates transcription of the IgM H-chain gene, and GCN5 deficiency decreased membrane-bound and secreted forms of IgM proteins [42]. GCN5 also directly activates expression of the TF IRF4, which is required for B-cell differentiation [43]. PCAF acetylates the TF E2A, which plays a major role in the differentiation of B lymphocytes [44].
HDACs also appear to play a role in signaling from the B-cell receptor (BCR). During BCR activation, HDACs 5 and 7 are phosphorylated by protein kinases D1 and D3 and exported from the nucleus, suggesting a link between BCR function and epigenetic regulation of chromatin structure [45].
A major regulator of B-cell differentiation is the TF BCL6, which represses a set of target genes during proper germinal center (GC) development [46]. BCL6 also serves as an anti-apoptotic factor during an immune response, which enables DNA-remodeling processes to occur without eliciting an apoptotic DNA damage response [47, 48]. To achieve GC-specific gene expression, BCL6 is recruited to a large repressor complex that contains HDAC4, 5, and 7, and localizes to the nucleus to regulate its target genes [49]. Treatment of cells with an HDACi results in hyper-acetylation of BCL6, which derepresses expression of BCL6 target genes involved in lymphocyte activation, differentiation, and apoptosis [50, 51].
In B cells, HDAC1 and 2 play a key, redundant role in cell proliferation and at certain stages of development. That is, in early B cells the combined KO of HDAC1 and 2 results in a loss of further B-cell development and the few surviving pre-B cells undergo apoptosis due to a cell cycle block in G1, whereas individual KOs of these HDACs has no effect [52]. In mature B cells, the combined KO of HDAC1 and 2 has no effect on cell survival or function in the resting state, but these double KO cells fail to proliferate in response to lipopolysaccharide and IL-4 [52].
HATs and HDACs in T-cell development and function
HATs and HDACs also play roles in T-cell development and function. For example, the HAT p300 is important for the expression of chemokine CCR9, which is expressed in thymocytes during their migration and development into mature T cells [53]. Early in this developmental process, NOTCH signaling prevents p300 recruitment to, and acetylation of, core histones at two CCR9 enhancers, thus reducing CCR9 expression [53]. This NOTCH-dependent repression of CCR9 occurs via effects on p300 in multipotent progenitor cells and is also observed in T-lymphoma cell lines [53].
Thymus-specific deletion of the bromodomain-containing protein BRD1, which is a subunit of the HAT HBO1 complex [54], alters the pattern of CD4/CD8 expression in thymocytes and decreases the abundance of CD8+ mature T cells in the periphery [55]. Furthermore, the HBO1-BRD1 complex is responsible for activating CD8 expression by increasing global acetylation of H3K14 in developing T cells [55].
T cell-specific KO of HDAC1 does not affect late T-cell development or the number of T cells in the periphery [56]. The lack of an effect is likely due to compensation by HDAC2, whose expression is increased when HDAC1 is inactivated [56]. Moreover, T cell-specific KO of both HDAC1 and 2 results in arrested T-cell development [57], similar to what is seen in HSCs and early B cells (see above). Nevertheless, T cell-specific KO of HDAC1 alone does cause an increased Th2-type inflammatory response in a mouse model of asthma, which is characterized by elevated expression of IL-4, IL-5, and IL-10, suggesting that HDAC1 represses cytokine production in activated T cells and during T effector (Teff) cell differentiation [56]. Of note, the HDAC1-deficient increased expression of IL-4 in T cells is seen only after several rounds of cell division, suggesting that the effect of HDAC1 on IL-4 expression occurs via an epigenetic mechanism, and that the removal of repressive marks occurs during DNA replication [56]. In T cells, an HDAC1/mSIN3A complex represses IL-2 expression [58], and during T-cell activation, HDAC1-mediated repression of IL-2 is relieved by phosphorylation of mSIN3A by CDK5, which disrupts the formation of the HDAC1/mSIN3A complex [58].
HDAC6-null mice exhibit normal B-cell development, but have reduced IgM and IgG levels following antigen stimulation [59]. This defect may be due to the role of HDAC6 in immune synapse formation and T-cell migration [60, 61].
In developing T cells, the interaction of class 2 HDACs with the TF MEF2D plays a major role in regulating T-cell receptor (TCR)-mediated apoptosis during negative selection of T cells with a TCR that interacts with self-antigen. For example, HDAC7 is recruited to the NUR77 promoter by MEF2D, where it acetylates chromatin and represses the expression of this apoptotic regulator [62]. In T cells undergoing negative selection, HDAC7 or class 2 HDACs become phosphorylated near their N-termini by protein kinase D, which allows recognition by 14-3-3, disruption of the interaction with MEF2D, and results in nuclear export of the repressive HDAC [62-64]. In addition to its role in negative selection, HDAC7 regulates the expression of genes involved in positive thymic selection. For example, HDAC7 KO and mutation studies in mouse thymocytes have shown that transcription of HDAC5 is regulated by MEF2D but not by NUR77; thus, HDAC5 may be a direct target for transcriptional regulation by HDAC7 during positive, but not negative T-cell selection [65, 66]
Class 4 HDAC11 represses the expression of IL-10 in antigen-presenting cells (APCs) by interacting with the distal region of the IL-10 promoter [67]. HDAC11 localization at the IL-10 distal promoter is coupled to increased binding of the transcriptional repressor PU.1 at the distal promoter and decreased acetylation of histones H3 and H4 at the proximal IL-10 promoter [67]. APCs that overexpress HDAC11 are able to restore the responsiveness of tolerant CD4+ T cells [67].
HATs and HDACs in the development of T-regulatory cells
T-regulatory cells (Tregs) play an important role in limiting T-cell immune responses, and HATs and HDACs have a variety of roles in Treg function. p300 and other HATs maintain the stability and function of Tregs by acetylating the TF FOXP3, whose transcriptional output is required for Treg-mediated immunosuppression [68]. FOXP3 expression in Tregs can be either positively or negatively regulated by the TF KLF10 through its association with PCAF or SIN3-HDAC1, respectively [69]. Interestingly, p300-deficient Tregs show many of the same defects in activity, survival and proliferation that occur in FOXP3-deficient Tregs [68], suggesting that the effects of p300 deficiency on Treg function are due to a reduction in FOXP3 activity. FOXP3 has also been found in a transcriptional regulatory complex with TIP60, HDAC7, HDAC9, and other proteins [70].
The acetylation of FOXP3 by either p300 or TIP60 both protects FOXP3 from degradation and increases its DNA-binding activity, and as a result, p300- or TIP60-deficient Tregs have defects in activity, survival, and proliferation [68, 71]. Deleting either p300 or CBP in FOXP3+ Tregs in mice does not affect the overall proportion of T cells under basal conditions, and thus, these two HATs appear to have redundant roles in Treg production under resting conditions [72, 73]. When the Tregs are activated, however, p300- or CBP-deficient FOXP3+ Tregs undergo apoptosis, are unable to suppress homeostatic Teff cell proliferation, and reject transplanted allografts [72, 73]. Mice with simultaneous Treg-specific deletion of p300 and CBP develop severe autoimmunity, as both p300 and CBP interact not only with FOXP3, but also with many FOXP3-regulating TFs including NFAT, STAT1, FOXO1, FOXO3, NF-κB, RUNX1, and STAT5 [72, 74-78]. Nevertheless, p300 and CBP also have distinct roles in Tregs; for example, only p300 is required for efficient GATA-3 expression, which is important for FOXP3 expression and Treg accumulation [72].
Countering the HATs, the HDACs deacetylate FOXP3, which reduces Treg development and immunosuppressive function, and also provides a therapeutic target for enhancing immunosuppressive (and potentially anti-tumor) activity in patients [79-81]. FOXP3 can be deacetylated by certain HDACs (i.e., HDAC3, 6, 7, 9 and SIRT1), which decreases FOXP3 protein levels and activity [80, 82, 83]. Of note, HDAC6, which is normally cytoplasmic, translocates to the nucleus of some Tregs where it can deacetylate FOXP3 [81]. Treg-specific deletion of these HDACs or treatment with HDACi has been shown to enhance immunosuppressive activity and Treg function [79]. Taken together, these results suggest that acetylation of FOXP3 favors Treg development. However, while Treg development is important in limiting host autoimmunity, it may also reduce host immune responses and anti-tumor activity. Thus, inhibition of FOXP3 acetylation is a promising anti-tumor strategy [73].
Regulation of Immune Cell-Related TFs by Acetylation
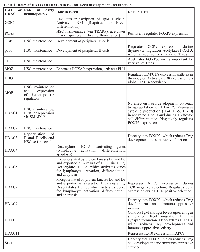
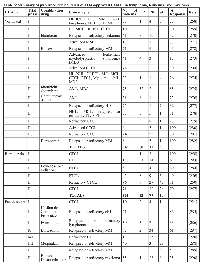
The activities of several TFs that play key roles in immune responses are affected directly and indirectly by HATs/HDACs. In most cases, the ability of CBP/p300 to acetylate a given TF and affect its activity has been investigated (summarized in Table 2). There are four general ways that direct acetylation has been shown to affect TF function: 1) lysine acetylation can increase protein stability by blocking ubiquitination of the same lysines that promote proteasome-mediated degradation; 2) lysine acetylation within the DNA-binding domain can decrease the ability of the TF to bind DNA; 3) lysine acetylation can increase (or decrease) protein-protein interactions with TF regulators; and 4) acetylated lysines on TFs can serve as a docking domain for the bromodomain of HATs, which can increase their transactivation activity [18]. For example, NF-κB and STAT subunits are important regulators of B- and T-cell development and function. Both of these TFs undergo acetylation/deacetylation at several lysines, and acetylation at different residues can positively or negatively impact their activity in distinct ways depending on the lysine residue [84, 85]. Furthermore, the activity of NF-κB and STAT can be indirectly affected by acetylation, for example, by acetylation of their specific co-activators, the TFs that interact with them, or histones at their target gene promoters. Moreover, the ability of acetylation to affect NF-κB and STAT activity can depend on the specific target gene studied.
In a small number of cases, it is known how acetylation alters the activity of a TF in a way that affects B- or T-cell function. As described above, the TF FOXP3 is a direct substrate of p300 and other HATs, and acetylation of FOXP3 plays a key role in Treg development and maintenance [68]. Acetylation increases FOXP3 activity by stabilizing the protein and enhancing its DNA-binding activity at certain promoters [68].
BCL6 is a transcriptional repressor that is essential for GC formation and lymphocyte function and proliferation [86, 87]. p300 can directly bind to and acetylate BCL6, which interferes with its ability to bind HDAC-containing complexes and consequently inactivates its repressor activity [50]. CBP/p300 mutant proteins found in some diffuse large B-cell lymphomas (DLBCLs) show a reduced ability to acetylate BCL6 [88], and therefore such lymphoma cells have increased BCL6 activity, which is related to the oncogenic state of these cells.GATA-3 plays a key role in T-cell differentiation and survival, and acetylation has been shown to increase GATA-3 transactivation activity [89]. Moreover, overexpression of an acetylation-defective GATA3 protein affects T-cell homing to lymph nodes and increases T-cell survival after antigen stimulation [89].
HATs and HDACs in B- and T-cell Malignancy
Given their broad role in control of lymphoid cell gene expression and TF activity, it is not surprising that misregulated acetylation is found in many cancers. As described in more detail below, in B- and T-cell cancers one often finds gene deletions and mutations that inactivate or reduce HAT activity (e.g., in CBP/p300) or overexpression of non-mutant forms of HDACs. As a consequence, reduction of global histone and TF acetylation appears to be correlated with B- and T-cell proliferation and survival, whereas increased acetylation is associated with B- and T-cell tumor growth arrest and cell death.
Mutations of HATs in B- and T-cell leukemia/lymphoma
Although chromosomal translocations involving p300, CBP and MYST are well-documented in acute myeloid leukemia [90], they have not been found in B- and T-cell malignancies. However, other types of HAT gene mutations are common in certain types of B- and T-cell cancers. Namely, the genes encoding CBP and p300 harbor point mutations or deletions in approximately 20–40% of DLBCL [88, 91, 92], about 70% of follicular lymphomas (FL) [93], and less frequently in T-cell leukemia, acute lymphoblastic leukemia (ALL) and myelodysplastic syndrome [94, 95]. The TIP60 gene frequently suffers mono-allelic loss and reduced expressed in several types of B-cell lymphoma [95]. Moreover, our analysis of the Cancer Cell Line Encyclopedia (CCLE) database [96] finds that mutations in CBP/p300 and other HATs (especially MORF) are common in a variety of B- and T-cell cancer cell lines (Table 3). With CBP and p300, the majority of these lymphoma mutations occur within or near the HAT domain or introduce frame-shifts or stop codons N-terminal to the HAT domain (see Figure 2). Thus, many of the CBP/p300 mutations found in DLBCL and FL are predicted to reduce acetyltransferase activity [88]. Indeed, several of these point mutations have been demonstrated to impair the affinity of CBP for acetyl-CoA and consequently compromise the ability of CBP to acetylate the TFs BCL6 and p53 [88]. Of note, acetylation of BCL6 decreases its gene repressing activity, whereas acetylation of p53 is required for its gene activation function (Table 2) [50, 97]. Thus, DLBCL cells with HAT gene mutations have higher levels of active BCL6 and lower levels of active p53 [88], consistent with decreased acetylation being associated with increased tumor cell growth.
In contrast to the more common point mutations, genomic alterations that completely remove the HAT domain in CBP or p300 are present in a minority of DLBCL and FL tumors and cell lines [88, 93]. Expression of C-terminally truncated CBP/p300 proteins missing the HAT domain has been demonstrated in some DLBCL cell lines [88, 98, 99]. A variety of evidence suggests that these HAT-deficient p300 mutants play an active role in lymphomagenesis. First, expression of the HAT-deficient p300 proteins is preferentially retained in these cell lines, whereas the wild-type allele is silenced [98, 99]. Second, knockdown of p300 mutant protein expression reduces the growth of some DLBCL cell lines [98, 99]. Third, HAT-deficient p300 mutants localize to sites of active transcription in cell nuclei, interact with the lymphomagenic TF REL, and affect transcription of REL target genes [98-100]. Moreover, expression of a HAT-deficient mutant of p300 increases the proliferation of HSCs that lack wild-type p300 [27]. DLBCL cell lines that express HAT-defective p300 mutants have generally lower levels of histone H3 acetylation at K14 and K18 [99]. Low levels of H3K14 and H3K18 acetylation have been associated with proliferation in other cell types [101], and CBP/p300 catalyze nearly all H3K18 acetylation in mice [102]. Thus, even though they are defective for HAT activity, C-terminally truncated p300 proteins appear to contribute to B-cell transformation, at least in part, by acting as aberrant scaffolds that organize altered transcription complexes at a variety of gene promoters/enhancers to cause a broad-range of transcriptional deregulation. For example, we have previously suggested that dampening of global REL/NF-κB-dependent gene transcription is one oncogenic effect of p300 mutants in DLBCL [99].
Similar to the effects of mutations on HAT activity in lymphoma, the HBZ protein of Human T-cell Leukemia Virus type 1 (HTLV-1) binds to and inactivates the HAT domains of CBP and p300, and consequently reduces cellular levels of H3K18 acetylation [103, 104]. Thus, inhibition of CBP/p300 HAT activity may also be important for HTLV1-induced T-cell leukemia.
Interestingly, the TF BCL6, which is upregulated in and required for the growth of approximately 70% of DLBCLs [105], appears to be a direct transcriptional repressor of the p300 gene [106]. Furthermore, induced expression of p300 is required for the anti-proliferative effects of BCL6 inhibitors on DLBCL cell lines [106]. Consequently, DLBCL cell lines with defective p300 proteins are resistant to the anti-growth effects of BCL6 inhibitors, and in these cell lines, HDACi synergize with BCL6 inhibitors for inhibition of DLBCL cell growth [106].Overall, there are no good mouse models for HAT gene mutations in B- and T-cell malignancy. In one report [107], a single mouse reconstituted with CBP-null HSCs developed a thymic lymphoma that arose from the CBP-null cells, but that mouse has not been further characterized. Based on the inactivation of the wild-type EP300 allele in DLBCLs containing certain p300 mutations [98, 99], expression of truncated or mutant p300 proteins (from human DLBCLs) in p300-/- B-cell precursors may lead to B-cell malignancy in a transgenic mouse model.
HDAC dysregulation in B- and T-cell lymphoma/leukemia
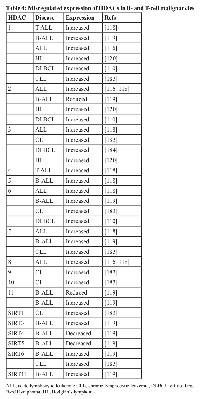
Unlike the case with HATs, mutations in genes encoding HDACs have not been found in any B- and T-cell malignancies. However, HDACs have been reported to have altered (usually increased) expression in a variety of B- and T-cell malignancies, including DLBCL, FL, and chronic lymphocytic leukemia (CLL) (Table 4). For example, HDAC1 is overexpressed in some T-cell lymphomas [108-112], while HDAC6 has been reported to be both overexpressed [110, 113] and underexpressed [114] in DLBCL.
At this point, two of the most relevant questions are whether altered expression of a specific HDAC contributes to the growth or survival of the tumor cells (and how it does so) and whether altered HDAC expression can be prognostic for therapy. In a smattering of cases, there are data addressing these questions, but the overall picture is still not clear. Inhibition of HDAC8 induces apoptosis in T cell-derived lymphoma and leukemic cells, but not in solid tumors [115]. High HDAC4 expression is associated with a poor response to prednisone in ALL, and siRNA-mediated inhibition of HDAC4 has been shown to sensitize a T-ALL cell line to etoposide-induced cell death [116]. Moreover, the interaction of HDAC4 with the leukemic PLZF-RARα fusion protein contributes to oncogenesis because it is required for the repression of differentiation-associated genes [117].
In childhood acute lymphoblastic leukemia (ALL), high HDAC3 expression has been associated with a better prognosis, whereas overexpression of HDAC7 and 9 have been associated with a poorer prognosis [118]. A study of over 200 adult CLL B-cell tissue samples reported that overexpression of HDAC7 and 10 and underexpression of HDAC6 and SIRT3 are correlated with a poor prognosis [119]. HDAC6 overexpression correlates with a more favorable outcome in DLBCL, but with a negative outcome in peripheral T-cell lymphoma [110]. Although HDAC1, 2 and 3 are all overexpressed in Hodgkin’s lymphoma tissue samples, only high HDAC1 expression is correlated with a worse outcome [120].
As with HATs, there are no good mouse models for the role of HDACs in cancer. Based on most evidence, it is unlikely that overexpression of any HDAC would, by itself, be oncogenic. Thus, one method for evaluating the molecular mechanisms by which increased HDAC expression contributes to oncogenesis might be to create transgenic mice with B and/or T cell-specific expression of a relevant HDAC (e.g., HDAC6) and cross such mice to other common transgenic mouse tumor models (e.g., Eµ-MYC mice). One could then determine whether increased HDAC expression leads to enhanced tumor development or if such mice develop chemo- or HDACi-resistant tumors.
HAT and HDAC Inhibitors in the Treatment of B- and T-cell Cancers
Given that mutations and dysregulation of HATs and HDACs occur in many B- and T-cell cancers, as well as their global effects on protein activity and gene expression, these enzymes have been investigated for therapeutic targeting. Below we discuss the types of compounds that have been found to inhibit HAT and HDAC activity, and examples of such molecules being used in the treatment of lymphoid cancer cells. Overall, HDACi have been more useful in such settings than HATi, and HDACi are being used in the clinic to treat lymphoid cell cancers.
HAT inhibitors (HATi)
Several types of compounds have been characterized as HAT inhibitors, including a variety of synthetic compounds and natural products and their derivatives. In general, such compounds are pan-HAT inhibitors or inhibitors of GCN5 or CBP/p300 [121, 122].
There are few reports of HATi as inhibitors of B- or T-cell cancers, and no HATi are currently FDA approved. Anacardic acid, found in cashew nuts, is a potent inhibitor of p300, PCAF, and TIP60 [123, 124]. Anacardic acid and derivatives have been shown to inhibit Jurkat T-cell leukemia cells at micromolar concentrations [125]. Of note, Jurkat cells have been shown to express two TIP60 variants, including one with a deleted HAT domain [126]. The natural products gallic acid and curcumin have both been shown to act as HAT inhibitors [127, 128], and can induce proliferation arrest and apoptosis in lymphoma cells [129, 130]. However, gallic acid and curcumin are not especially potent inhibitors of lymphoma cell growth and both have many protein targets [131, 132]; thus, it is not clear that their effects on lymphoma cell growth are due to their anti-HAT activity. The synthetic compound C646 is a specific p300 inhibitor, however, it was not especially effective against leukemia cell lines in a screen of the National Cancer Institute (NCI) 60-cell line panel [133].
HDAC inhibitors (HDACi)
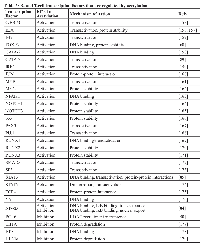
Given that increased HDAC expression and activity is found is many lymphoid malignancies (see above), it is perhaps not surprising that HDACs should emerge as targets for therapy. In contrast to HATi, HDACi have been extensively studied for anti-cancer activity. Indeed, since 2001, a number of HDACi have been used in the clinic for the treatment of various cancers, including B- and T-cell cancers [134]. Alone or in combination with other anti-cancer agents, a variety of HDACi have been shown to induce apoptosis in many different types of B- and T-cell lymphoma and leukemia cell lines (Table 5). Following from those studies, several HDACi have been tested clinically for the treatment of such human cancers, including cutaneous T-cell lymphoma (CTCL), DLBCL, multiple myeloma (MM), FL, Hodgkin’s lymphoma (HL), and several others (Table 6), and as of April 2015, there are at least 12 ongoing clinical trials testing HDACi alone or in combination with other cancer therapeutics for the treatment of several B- and T-cell malignancies (Table 7).
HDACi fall into five main classes, based in part on their chemical structures and in part on their specificity. These include the following: 1) hydroxyamic acids, 2) cyclic tetrapeptides, 3) benzamides, 4) ketones, and 5) aliphatic acids. In addition, HDACi can have broad-based pan-HDAC inhibitory activity, have class specificity, or even isozyme specificity. Currently, four HDACi have received FDA approval for clinical use. The first two FDA-approved inhibitors are the pan-HDACi vorinostat (aka suberoylanilide hydroxamic acid [SAHA]), which is available as an oral medication, and the class I HDACi romidepsin (a bacterial cyclic peptide), which is administered intravenously. HDACi treatment is especially effective in the treatment of CTCL, with favorable response rates (from a number of trials) of approximately 70% when using romidepsin (Table 6). Although it is not known why CTLC responds well to HDACi treatment, increased expression of HDAC2 and histone H4 acetylation have been correlated with aggressive CTCL [110]. The HDACi belinostat was approved in 2014 for relapsed and refractory peripheral T-cell lymphomas [135]. Finally, the HDACi panobinostat, another hydroxamate, has been approved by the FDA for refractory multiple myeloma [136]. Because of the role of HDACs in normal immune cell function, one problem with using HDACi treatment for anti-cancer therapy is the concomitant suppression of host immune responses required for anti-tumor therapy (see also HATs and HDACs in development of T-regulatory cells section above) [137].
In the simplest scenario, HDACi treatment increases the level of acetylation of histones on chromatin, thereby increasing gene expression, and HDACi also increase the acetylation of non-histone proteins. For lymphoid cell TFs, increased acetylation can either increase or decrease their activity (Table 2). Because of the myriad effects of acetylation/deacetylation on gene expression and protein activity, it is almost certain that the effects of HDACi on tumor cell growth and survival are not through single or even a small number of targets. Moreover, the effects of HDACi would be expected to vary among tumor cell types, within a given tumor type, and, due to tumor cell heterogeneity, even within a given tumor.
Consistent with those hypotheses, treatment of CTCL cell lines with vorinostat showed that HDACi treatment leads to hyperacetylation of all core histones, which is associated with changes in the expression of genes involved in regulation of the G1/S and G2/M transitions, apoptosis, anti-proliferation, and MAPK signaling [138]. Overall, gene expression profiling showed that up to 22% of genes are altered by HDACi as early as four hours post treatment in several cell types [139-141]. Nevertheless, there does appear to be a common set of genes that change expression in response to HDACi treatment, and these genes include several cyclins, the cell-cycle inhibitor p21, p53, BAX, BCL2, MYC, PKCδ, ICAM-1, IL-6 receptor, IL-2, IL-8, IL-10, VEGF, NOTCH, GADD45 and GADD45, TGF receptor, CTP synthase, and TYMS (reviewed by [17, 134, 142]. At least in part, HDACi induce cell-cycle arrest by causing accumulation of hyperacetylated p53, which then induces expression of p21, leading to inhibition of cyclins D and A, which are required for cell-cycle progression (reviewed by [134, 142]). However, because CTCL tumors typically grow quite slowly in patients, it is unclear how the reported effects of HDACi on cell-cycle progression in rapidly growing cell lines in vitro reflect its effects on CTCL tumors in vivo.
HDACi treatment of tumor cells has been frequently linked to the modulation of BCL2 family expression to favor a pro-apoptotic expression pattern (reviewed by [134, 142, 143]). In many cases, HDACi-induced apoptosis occurs via increased expression of pro-apoptotic BCL2 family members BIM, BAX, PUMA, and NOXA (reviewed by [142, 144, 145]). Moreover, resistance to HDACi-induced apoptosis can be achieved in DLBCL cells lines by artificial or induced expression of anti-Entinostat (MS-275)Benzamide HDAC1-3 Phase I,
II ALL, AML, CML, HL, MM Azacitadine, Imatinib, Isotretinoin, Sargramostim, Sorafenib, Rapamycin, Rituximab[142, 237-241]Mocetinostat (MGCD-0103) Benzamide HDAC1-3, 10, 11 Phase I, II AML, CLL HL, NHL 5-azacitadine, Bortezomib, Docetaxel, Gemcitabine, GX15-070 [242-250]Romidepsin (FK228)Cyclic tetrapeptide Class 1, 2, 4 Phase I,
II, III ALL, AML, CLL, CTCL, DLBCL, MM, MCL, NHL, PTCL, SLL Bortezomib, Carboplatin, Cyclophosphamide, Decitadine, Etoposide, Ifosfamide, Prednisone, Rituximab, Vincrstine [206, 210, 223, 251-260]Apicidin Cyclic tetrapeptide HDAC1, 3 APL, CML Imatinib, TRAIL[142, 261-264] Nicotinamide Vitamin B member Sirtuins CLL[265]Tenovin-6 (TV-6)Small Molecule SIRT1 CML Imatinib[266]Amurensin G Natural Product SIRT1 TRAIL-resistant leukemia [267]
Cell types: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; BL, Burkitt’s lymphoma; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; CTCL, cutaneous T-cell lymphoma; DLBCL, diffuse large B-cell lymphoma; EBV+ BL, Epstein-Barr virus-positive BL; HL, Hodgkin’s lymphoma; MCL, mantle cell lymphoma; MM, multiple myeloma; NHL, non-Hodgkin’s lymphoma; PTCL, peripheral T-cell lymphoma; SLL, small lymphocytic lymphoma.Drug type: 17-AAG (Hsp90 inhibitor); ABT-737 (BH3-mimetic); ATRA (all-trans retinoic acid); azacitidine (DNA methyltransferase inhibitor); bexarotene (antineoplastic agent); bortezomib (proteasome inhibitor); cambinol (sirtuin inhibitor); carboplatin (antineoplastic agent); carfilzomib (proteasome inhibitor); cisplatin (alkylating agent); cladribine (adenosine deaminase inhibitor); cyclophosphamide (alkylating agent); cytarabine (DNA synthesis inhibitor); decitabine (DNA methyltransferase inhibitor); dexamethasone (glucocorticoid steroid); docetaxel (anti-mitotic agent); eltrombopag (thrombopoeitin receptor agonist); enzastaurin (PKCβ inhibitor); etoposide (topoisomerase inhibitor); everolimus (mTOR inhibitor); EX527 (sirtuin inhibitor); gemcitabine (nucleoside analog); GX15-070 (BH3-mimetic); idarubicin (topoisomerase II inhibitor); imatinib (tyrosine kinase inhibitor); ifosfamide (alkylating agent); isotretinoin (retinoic acid analog); lenalidomide (tumor necrosis factor [TNF] inhibitor); melphalan (alkylating agent); NPI-0052 (proteasome inhibitor); pazopanib (tyrosine kinase inhibitor); pemetrexed (folate antimetabolites); pioglitazone (thiazolidinedione); prednisone (glucocorticoid prodrug); rituximab (anti-CD20 antibody); sargramostin (recominant GM-CSF); sirtinol (sirtuin inhibitor); sorafenib (tyrosine kinase inhibitor); temozolomide (alkylating agent); TRAIL (TNF-related apoptosis-inducing ligand ); vincristine (mitotic inhibitor).
II Refractory CTCL 139 2 22 29 17[291]II Relapsed or refractory HL 129 5 30 71 27[292]II Melphalan, Thalidomide, Prednisone Relapsed or refractory MM 31 2 10 11 39[293]III Bortezomib, Dexamethasone Relapsed or refractory MM 387 42 216 65 67[136]TOTALS 955 59 369 217 Belinostat I NHL, HL, MM, CLL 16 5[213]II Relapsed or refractory CTCL 29 3 1 10 4[294]II Recurrent PTCL 24 2 4 4 6[294]III Relapsed or refractory CTCL 120 11 15 26[135]TOTALS 189 16 20 19
CR, complete response; PR, partial response; SD, stable disease.
apoptotic protein BCL-XL [146]. Increased activity of BCL2, thioredoxin, and CHK1 has also been associated with the development of HDACi resistance in lymphoma [147].There is considerable interest in identifying markers that can predict responsiveness to HDACi therapy [148]. Markers that have been reported to predict better response to HDACi treatment include high levels of shuttling protein HR23B [149] and several induced mRNAs, including cyclin D1 [150] for CTCL and CDKN1A [151] for DLBCL. Interestingly, it has been reported that DLBCLs with mutations in p300 or CBP are more responsive to HDACi treatment [152-154], suggesting that decreased HAT activity makes HDACi treatment more successful and that combined treatment with HATi and HDACi could be a useful strategy.
One note of caution in the use of HDACi is the finding that loss of HDAC1/2 activity by gene KO in mouse T cells has been reported to lead to T-cell malignancy, and these malignant cells show increased expression of the oncoprotein MYC and aneuploidy [57].
CONCLUSIONS AND PERSPECTIVES
The role of acetylation in regulating chromatin structure, gene expression, and protein activity will undoubtedly continue to receive much attention. Given the complex signaling and gene expression changes that occur in B- and T-cell development, there is much more to be learned about the role of regulated acetylation in these processes.
Although the use of HATi for therapy is at an early stage, HDACi treatment is likely to continue for the treatment of B- and T-cell malignancies and certain immune diseases. Thus, a deeper understanding of the proteins, genes, and pathways affected by deregulated acetylation will be crucial to applying HDACi in the clinic. Given the wide range of transcriptional regulators affected by acetylation, there are clearly many targets affected by HDACi treatment and these targets no doubt vary among different cancers. Thus, HDACi may be most effective when combined with therapeutics that target specific pathways in individual cancers. The use of HDACi in combination with other therapeutics is a strategy that is being used in many ongoing clinical trials (Table 7), and the ability to prescribe appropriate combined HDACi-targeted drug regimens will improve as better ways are developed to molecularly profile pathways that are driving individual cancers.
ACKNOWLEDGMENTS
We thank Drs. Gerald Denis and Adam Lerner for comments on the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
FUNDING
L.H. and R.C.T. were supported by NHLBI Hematology training grant T32 HL007501. L.H. was also supported by NSF GK-12 grant DGE-0947950. Research in our lab on the role of HATs and HDACs in B-cell lymphoma was supported by NIH grant CA047763, NIH ARRA supplement CA047763-21S3 (both to T.D.G.), and administrative funds from Boston University.


- 1. Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell. 1996; 84: 843851. [PubMed]
- 2. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science. 1996; 272: 408-411. [PubMed]
- 3. Acetylation and deacetylation of non-histone proteins. Gene. 2005; 363: 1523. [PubMed]
- 4. Transcriptional repression by UME6 involves deacetylation of lysine 5 of histone H4 by RPD3. Nature. 1998; 392: 831-835. [PubMed]
- 5. Structure and function of histone acetyltransferases. Cell Mol Life Sci. 2001; 58: 693-703. [PubMed]
- 6. The structural basis of protein acetylation by the p300/CBP transcriptional coactivator. Nature. 2008; 451: 846-850. [PubMed]
- 7. Structure and chemistry of the p300/CBP and Rtt109 histone acetyltransferases: implications for histone acetyltransferase evolution and function. Curr Opin Struct Biol. 2008; 18: 741-747. [PubMed] https://doi.org/10.1016/j.sbi.2008.09.004.
- 8. Structure of the GCN5 histone acetyltransferase bound to a bisubstrate inhibitor. Proc Natl Acad Sci USA. 2002; 99: 14065-14070. [PubMed] https://doi.org/10.1073/pnas.222373899.
- 9. Structure and functions of the GNAT superfamily of acetyltransferases. Arch Biochem Biophys. 2005; 433: 212-226. [PubMed]
- 10. Histone acetyltransferase activity of p300 is required for transcriptional repression by the promyelocytic leukemia zinc finger protein. Mol Cell Biol. 2005; 25: 5552-5566. [PubMed] https://doi.org/10.1128/MCB.25.13.5552-5566.2005.
- 11. Chromatin modifications and their function. Cell. 2007; 128: 693-705. [PubMed]
- 12. Activation of AML1-mediated transcription by MOZ and inhibition by the MOZ-CBP fusion protein. The EMBO journal. 2001; 20: 7184-7196. [PubMed] https://doi.org/10.1093/emboj/20.24.7184.
- 13. MOZ is essential for maintenance of hematopoietic stem cells. Genes Dev. 2006; 20: 13211330. [PubMed] https://doi.org/10.1101/gad.1393106.
- 14. MOZ and MORF histone acetyltransferases interact with the Runtdomain transcription factor Runx2. Oncogene. 2002; 21: 2729-2740. [PubMed]
- 15. ING tumor suppressor proteins are critical regulators of chromatin acetylation required for genome expression and perpetuation. Mol Cell. 2006; 21: 51-64. [PubMed]
- 16. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer. 2009; 9: 615-630. [PubMed] https://doi.org/10.1038/nrc2695.
- 17. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003; 370: 737-749. [PubMed] https://doi.org/10.1042/BJ20021321.
- 18. Class II histone deacetylases: from sequence to function, regulation, and clinical implication. Mol Cell Biol. 2005; 25: 2873-2884. [PubMed] https://doi.org/10.1128/MCB.25.8.2873-2884.2005.
- 19. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat Rev Mol Cell Biol. 2008; 9: 206-218. [PubMed] https://doi.org/10.1038/nrm2346.
- 20. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell. 2009; 138: 1019-1031. [PubMed] https://doi.org/10.1016/j.cell.2009.06.049.
- 21. Crystal structure of a SIR2 homolog-NAD complex. Cell. 2001; 105: 269-279. [PubMed]
- 22. Deacetylase enzymes: biological functions and the use of small-molecule inhibitors. Chem Biol. 2002; 9: 3-16. [PubMed]
- 23. The SMRT and N-CoR corepressors are activating cofactors for histone deacetylase 3. Mol Cell Biol. 2001; 21: 6091-6101. [PubMed] https://doi.org/10.1128/MCB.21.18.6091-6101.2001.
- 24. Sirtuins: nodes connecting aging, metabolism and tumorigenesis. Curr Pharm Des. 2014; 20: 1614-1624. [PubMed]
- 25. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem. 2005; 280: 13560-13567. [PubMed]
- 26. Gene dose-dependent control of hematopoiesis and hematologic tumor suppression by CBP. Genes Dev. 2000; 14: 272-277. [PubMed]
- 27. Systematic in vivo structure-function analysis of p300 in hematopoiesis. Blood. 2009; 114: 4804-4812. [PubMed]
- 28. Monocytic leukemia zinc finger protein is essential for the development of long-term reconstituting hematopoietic stem cells. Genes Dev. 2006; 20: 1175-1186. [PubMed] https://doi.org/10.1101/gad.1382606.
- 29. A transcription-factor-binding surface of coactivator p300 is required for haematopoiesis. Nature. 2002; 419: 738-743. [PubMed]
- 30. Differential roles of p300 and PCAF acetyltransferases in muscle differentiation. Mol Cell. 1997; 1: 35-45. [PubMed]
- 31. The ETS family transcription factor PU.1 is necessary for the maintenance of fetal liver hematopoietic stem cells. Blood. 2004; 104: 3894-3900. [PubMed]
- 32. Distinctive and indispensable roles of PU.1 in maintenance of hematopoietic stem cells and their differentiation. Blood. 2005; 106: 1590-1600. [PubMed] https://doi.org/10.1182/blood-2005-03-0860.
- 33. The histone acetyl transferase activity of monocytic leukemia zinc finger is critical for the proliferation of hematopoietic precursors. Blood. 2009; 113: 4866-4874. [PubMed] https://doi.org/10.1182/blood-2008-04-152017.
- 34. The histone acetyltransferase MOF is a key regulator of the embryonic stem cell core transcriptional network. Cell Stem Cell. 2012; 11: 163-178. [PubMed] https://doi.org/10.1016/j.stem.2012.04.023.
- 35. Histone acetyltransferase cofactor Trrap is essential for maintaining the hematopoietic stem/progenitor cell pool. J Immunol. 2009; 183: 6422-6431. [PubMed]
- 36. Sin3a-associated Hdac1 and Hdac2 are essential for hematopoietic stem cell homeostasis and contribute differentially to hematopoiesis. Haematologica. 2014; 99: 1292-1303. [PubMed] https://doi.org/10.3324/haematol.2013.092643.
- 37. Overlapping functions of Hdac1 and Hdac2 in cell cycle regulation and haematopoiesis. EMBO J. 2010; 29: 2586-2597. [PubMed] https://doi.org/10.1038/emboj.2010.136.
- 38. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009; 459: 55-60. [PubMed] https://doi.org/10.1038/nature07925.
- 39. Inhibition of endothelial ERK signalling by Smad1/5 is essential for haematopoietic stem cell emergence. Nat Commun. 2014; 5: 3431. [PubMed]
- 40. HDAC3 is essential for DNA replication in hematopoietic progenitor cells. J Clin Invest. 2013; 123: 3112-3123. [PubMed] https://doi.org/10.1172/JCI60806.
- 41. Global transcriptional coactivators CREB-binding protein and p300 are highly essential collectively but not individually in peripheral B cells. Blood. 2006; 107: 4407-4416. [PubMed] https://doi.org/10.1182/blood-2005-08-3263.
- 42. GCN5 is involved in regulation of immunoglobulin heavy chain gene expression in immature B cells. Gene. 2014; 544: 19-24. [PubMed]
- 43. GCN5 is essential for IRF-4 gene expression followed by transcriptional activation of Blimp-1 in immature B cells. J Leukoc Biol. 2014; 95: 399-404. [PubMed]
- 44. Regulation of E2A activities by histone acetyltransferases in B lymphocyte development. J Biol Chem. 2003; 278: 2370-2376. [PubMed]
- 45. Essential role for protein kinase D family kinases in the regulation of class II histone deacetylases in B lymphocytes. Mol Cell Biol. 2006; 26: 1569-1577. [PubMed] https://doi.org/10.1128/MCB.26.4.1569-1577.2006.
- 46. Differentiation of germinal center B cells and follicular helper T cells as viewed by tracking Bcl6 expression dynamics. Immunol Rev. 2012; 247: 120-132. [PubMed]
- 47. JunD/AP-1 and STAT3 are the major enhancer molecules for high Bcl6 expression in germinal center B cells. Int Immunol. 2006; 18: 1079-1089. [PubMed]
- 48. BCL6: master regulator of the germinal center reaction and key oncogene in B cell lymphomagenesis. Adv Immunol. 2010; 105: 193-210. [PubMed]
- 49. Class II histone deacetylases are directly recruited by BCL6 transcriptional repressor. J Biol Chem. 2002; 277: 22045-22052. [PubMed]
- 50. Acetylation inactivates the transcriptional repressor BCL6. Nat Genet. 2002; 32: 606-613. [PubMed]
- 51. Mutations of the BCL6 protooncogene disrupt its negative autoregulation in diffuse large B-cell lymphoma. Blood. 2003; 101: 2914-2923. [PubMed]
- 52. Histone deacetylases 1 and 2 act in concert to promote the G1-to-S progression. Genes Dev. 2010; 24: 455-469. [PubMed] https://doi.org/10.1101/gad.552310.
- 53. Repression of Ccr9 transcription in mouse T lymphocyte progenitors by the Notch signaling pathway. J. Immunol. 2015; 194: 3191-3200. [PubMed] https://doi.org/10.4049/jimmunol.1500914.
- 54. The Hbo1Brd1/Brpf2 complex is responsible for global acetylation of H3K14 and required for fetal liver erythropoiesis. Blood. 2011; 118: 2443-2453. [PubMed]
- 55. Histone acetylation mediated by Brd1 is crucial for Cd8 gene activation during early thymocyte development. Nat Commun. 2014; 5: 5872. [PubMed] https://doi.org/10.1038/ncomms6872.
- 56. Conditional deletion of histone deacetylase 1 in T cells leads to enhanced airway inflammation and increased Th2 cytokine production. J Immunol. 2010; 185: 3489-3497. [PubMed] https://doi.org/10.4049/jimmunol.0903610.
- 57. Histone deacetylase 1 and 2 are essential for normal T-cell development and genomic stability in mice. Blood. 2013; 121: 1335-1344. [PubMed] https://doi.org/10.1182/blood-2012-07-441949.
- 58. Cdk5 controls IL-2 gene expression via repression of the mSin3a-HDAC complex. Cell Cycle. 2015; 14: 1327-1336. [PubMed] https://doi.org/10.4161/15384101.2014.987621.
- 59. Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol Cell Biol. 2008; 28: 1688-1701. [PubMed] https://doi.org/10.1128/MCB.01154-06.
- 60. Lymphocyte chemotaxis is regulated by histone deacetylase 6, independently of its deacetylase activity. Mol Biol Cell. 2006; 17: 3435-3445. [PubMed] https://doi.org/10.1091/mbc.E06-01-0008.
- 61. HDAC6 deacetylase activity links the tubulin cytoskeleton with immune synapse organization. Immunity. 2004; 20: 417-428. [PubMed]
- 62. HDAC7, a thymus-specific class II histone deacetylase, regulates Nur77 transcription and TCR-mediated apoptosis. Immunity. 2003; 18: 687-698. [PubMed]
- 63. A role for the orphan steroid receptor Nur77 in apoptosis accompanying antigen-induced negative selection. Immunity. 1995; 3: 273-282. [PubMed]
- 64. Requirement for the orphan steroid receptor Nur77 in apoptosis of T-cell hybridomas. Nature. 1994; 367: 277-281. [PubMed]
- 65. Histone deacetylase 7 functions as a key regulator of genes involved in both positive and negative selection of thymocytes. Mol Cell Biol. 2007; 27: 5184-5200. [PubMed] https://doi.org/10.1128/MCB.02091-06.
- 66. Histone deacetylase 7 regulates cell survival and TCR signaling in CD4/CD8 double-positive thymocytes. J Immunol. 2011; 186: 4782-4793. [PubMed]
- 67. The histone deacetylase HDAC11 regulates the expression of interleukin 10 and immune tolerance. Nat Immunol. 2009; 10: 92-100. [PubMed] https://doi.org/10.1038/ni.1673.
- 68. Post-translational modification networks regulating FOXP3 function. Trends Immunol. 2014; 35: 368-378. [PubMed]
- 69. Differential coupling of KLF10 to Sin3-HDAC and PCAF regulates the inducibility of the FOXP3 gene. Am J Physiol Regul Integr Comp Physiol. 2014; 307: R608-620. [PubMed] https://doi.org/10.1152/ajpregu.00085.2014.
- 70. FOXP3 actively represses transcription by recruiting the HAT/HDAC complex. Cell Cycle. 2007; 6: 1432-1436. [PubMed]
- 71. FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc Natl Acad Sci USA. 2007; 104: 4571-4576. [PubMed] https://doi.org/10.1073/pnas.0700298104.
- 72. Two histone/protein acetyltransferases, CBP and p300, are indispensable for Foxp3+ T-regulatory cell development and function. Mol Cell Biol. 2014; 34: 3993-4007. [PubMed] https://doi.org/10.1128/MCB.00919-14.
- 73. Inhibition of p300 impairs Foxp3+ T regulatory cell function and promotes antitumor immunity. Nat Med. 2013; 19: 1173-1177. [PubMed] https://doi.org/10.1038/nm.3286.
- 74. Indispensable role of the Runx1-Cbfβ transcription complex for in vivo-suppressive function of FoxP3+ regulatory T cells. Immunity. 2009; 31: 609-620. [PubMed]
- 75. Development of Foxp3+ regulatory t cells is driven by the c-Rel enhanceosome. Immunity. 2009; 31: 932-940. [PubMed] https://doi.org/10.1016/j.immuni.2009.10.006.
- 76. IL-2 receptor β-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007; 178: 280-290. [PubMed]
- 77. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol. 2010; 11: 618627. [PubMed]
- 78. Runx-CBFβ complexes control expression of the transcription factor Foxp3 in regulatory T cells. Nat Immunol. 2009; 10: 1170-1177.
- 79. Inhibition of HDAC9 increases T regulatory cell function and prevents colitis in mice. Gastroenterology. 2010; 138: 583-594. [PubMed] https://doi.org/10.1053/j.gastro.2009.10.037.
- 80. Histone deacetylase inhibitors and transplantation. Curr Opin Immunol. 2007; 19: 589-595. [PubMed] https://doi.org/10.1016/j.coi.2007.07.015.
- 81. Histone deacetylases 6 and 9 and sirtuin-1 control Foxp3+ regulatory T cell function through shared and isoform-specific mechanisms. Sci Signal. 2012; 5: ra45. [PubMed] https://doi.org/10.1126/scisignal.2002873.
- 82. TGFβ and IL-6 signals modulate chromatin binding and promoter occupancy by acetylated FOXP3. Proc Natl Acad Sci USA. 2008; 105: 14023-14027.
- 83. FOXP3+ regulatory T cell development and function require histone/protein deacetylase 3. J Clin Invest. 2015: 125: 1111-1123. [PubMed] https://doi.org/10.1172/JCI83084.
- 84. Histone acetyltransferases are crucial regulators in NF-κB mediated inflammation. Drug Discov Today. 2011; 16: 504-511. [PubMed] https://doi.org/10.1016/j.drudis.2011.03.009.
- 85. Regulation of STAT signaling by acetylation. Cell Signal. 2013; 25: 1924-1931. [PubMed] https://doi.org/10.1016/j.cellsig.2013.05.007.
- 86. The BCL-6 protooncogene controls germinal-centre formation and Th2-type inflammation. Nat Genet. 1997; 16: 161-170. [PubMed]
- 87. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science. 1997; 276: 589-592. [PubMed]
- 88. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature. 2011; 471: 189-195. [PubMed] https://doi.org/10.1038/nature09730.
- 89. Acetylation of GATA-3 affects T-cell survival and homing to secondary lymphoid organs. EMBO J. 2000; 19: 4676-4687. [PubMed] https://doi.org/10.1093/emboj/19.17.4676.
- 90. Deregulated transcription factors in leukemia. Int J Hematol. 2011; 94: 134-141. [PubMed]
- 91. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat Genet. 2011; 43: 830-837. [PubMed] https://doi.org/10.1038/ng.892.
- 92. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011; 476: 298-303. [PubMed] https://doi.org/10.1038/nature10351.
- 93. Genetics of follicular lymphoma transformation. Cell Rep. 2014; 6: 130-140. [PubMed] https://doi.org/10.1016/j.celrep.2013.12.027.
- 94. Disease-related potential of mutations in transcriptional cofactors CREB-binding protein and p300 in leukemias. Cancer Lett. 2004; 213: 11-20. [PubMed]
- 95. CREBBP HAT domain mutations prevail in relapse cases of high hyperdiploid childhood acute lymphoblastic leukemia. Leukemia. 2012; 26: 17971803. [PubMed] https://doi.org/10.1038/leu.2012.60.
- 96. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012; 483: 603-607. [PubMed] https://doi.org/10.1038/nature11003.
- 97. Recruitment of p300/CBP in p53dependent signal pathways. Cell. 1997; 89: 1175-1184. [PubMed]
- 98. Histone acetyltransferase p300 is a coactivator for transcription factor REL and is C-terminally truncated in the human diffuse large B-cell lymphoma cell line RC-K8. Cancer Lett. 2010; 291: 237245. [PubMed] https://doi.org/10.1016/j.canlet.2009.10.018.
- 99. Histone acetyltransferase-deficient p300 mutants in diffuse large B cell lymphoma have altered transcriptional regulatory activities and are required for optimal cell growth. Mol Cancer. 2014; 13: 29. [PubMed] https://doi.org/10.1186/1476-4598-13-29.
- 100. A rearranged EP300 gene in the human B-cell lymphoma cell line RC-K8 encodes a disabled transcriptional co-activator that contributes to cell growth and oncogenicity. Cancer Lett. 2011; 302: 76-83. [PubMed] https://doi.org/10.1016/j.canlet.2010.12.018.
- 101. SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature. 2012; 487: 114-118. [PubMed] https://doi.org/10.1038/nature11043.
- 102. Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. EMBO J. 2011; 30: 249-262. [PubMed] https://doi.org/10.1038/emboj.2010.318.
- 103. The HTLV-1-encoded protein HBZ directly inhibits the acetyl transferase activity of p300/CBP. Nucleic Acids Res. 2012; 40: 5910-5925. [PubMed] https://doi.org/10.1093/nar/gks244.
- 104. An interaction between the human T cell leukemia virus type 1 basic leucine zipper factor (HBZ) and the KIX domain of p300/CBP contributes to the down-regulation of tax-dependent viral transcription by HBZ. J Biol Chem. 2008; 283: 23903-23913. [PubMed] https://doi.org/10.1074/jbc.M803116200.
- 105. B-cell lymphoma 6 and the molecular pathogenesis of diffuse large B-cell lymphoma. Curr Opin Hematol. 2008; 15: 381-390. [PubMed] https://doi.org/10.1097/MOH.0b013e328302c7df.
- 106. BCL6 repression of EP300 in human diffuse large B cell lymphoma cells provides a basis for rational combinatorial therapy. J Clin Invest. 2010; 120: 4569–4582. [PubMed] https://doi.org/10.1172/JCI42869.
- 107. Distinct roles for CREB-binding protein and p300 in hematopoietic stem cell self-renewal. Proc Natl Acad Sci USA. 2002; 99: 14789-14794. [PubMed] https://doi.org/10.1073/pnas.232568499.
- 108. Expression profile of histone deacetylase 1 in gastric cancer tissues. Jpn J Cancer Res. 2001; 92: 13001304. [PubMed] https://doi.org/10.1111/j.1349-7006.2001.tb02153.x.
- 109. Upregulation and nuclear recruitment of HDAC1 in hormone refractory prostate cancer. Prostate. 2004; 59: 177-189. [PubMed]
- 110. Histone deacetylase 1, 2, 6 and acetylated histone H4 in B- and T-cell lymphomas. Histopathology. 2009; 54: 688-698. [PubMed]
- 111. Histone deacetylase 3 (HDAC3) and other class I HDACs regulate colon cell maturation and p21 expression and are deregulated in human colon cancer. J Biol Chem. 2006; 281: 13548-13558. [PubMed]
- 112. Quantitation of HDAC1 mRNA expression in invasive carcinoma of the breast*. Breast Cancer Res Treat. 2005; 94: 11-16. [PubMed]
- 113. HDAC6 expression is correlated with better survival in breast cancer. Clin Cancer Res. 2004; 10: 6962-6968. [PubMed]
- 114. Expression of histone deacetylases in lymphoma: implication for the development of selective inhibitors. Br J Haematol. 2009; 147: 515-525. [PubMed] https://doi.org/10.1111/j.1365-2141.2009.07887.x.
- 115. Histone deacetylase 8 safeguards the human ever-shorter telomeres 1B (hEST1B) protein from ubiquitin-mediated degradation. Mol Cell Biol. 2006; 26: 5259-5269. [PubMed] https://doi.org/10.1128/MCB.01971-05.
- 116. The expression of histone deacetylase 4 is associated with prednisone poor-response in childhood acute lymphoblastic leukemia. Leuk Res. 2013; 37: 1200-1207. [PubMed]
- 117. HDAC4 mediates transcriptional repression by the acute promyelocytic leukaemia-associated protein PLZF. Oncogene. 2004; 23: 8777-8784. [PubMed]
- 118. Differential expression of HDAC3, HDAC7 and HDAC9 is associated with prognosis and survival in childhood acute lymphoblastic leukaemia. Br J Haematol. 2010; 150: 665-673. [PubMed]
- 119. HDAC isoenzyme expression is deregulated in chronic lymphocytic leukemia B-cells and has a complex prognostic significance. Epigenetics. 2012; 7: 1403-1412. [PubMed] https://doi.org/10.4161/epi.22674.
- 120. Class I histone deacetylases 1, 2 and 3 are highly expressed in classical Hodgkin’s lymphoma. Expert Opin Ther Targets. 2010; 14: 577-584. [PubMed]
- 121. Histone acetyl transferases as emerging drug targets. Drug Discov Today. 2009; 14: 942948. [PubMed]
- 122. Small molecule inhibitors of histone acetyltransferases and deacetylases are potential drugs for inflammatory diseases. Drug Discov Today. 2014; 19: 654-660. [PubMed]
- 123. Small molecule modulators of histone acetyltransferase p300. J Biol Chem. 2003; 278: 1913419140. [PubMed]
- 124. Inhibition of histone acetyltransferase activity by anacardic acid sensitizes tumor cells to ionizing radiation. FEBS Lett. 2006; 580: 43534356. [PubMed]
- 125. Characterization of novel inhibitors of histone acetyltransferases. Mol Cancer Ther. 2007; 6: 2391-2398. [PubMed]
- 126. Tip60 is a cell-type-specific transcriptional regulator. J Biochem. 2001; 129: 635-641. [PubMed]
- 127. Gallic acid suppresses lipopolysaccharide-induced nuclear factor-kappaB signaling by preventing RelA acetylation in A549 lung cancer cells. Mol Cancer Res. 2009; 7: 2011-2021. [PubMed]
- 128. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J Biol Chem. 2004; 279: 5116351171. [PubMed]
- 129. Developing curcumin into a viable therapeutic for lymphoma. Expert Opin Investig Drugs. 2009; 18: 57-67. [PubMed]
- 130. Gallic acid inhibits cell viability and induces apoptosis in human monocytic cell line U937. J Med Food. 2011; 14: 240-246. [PubMed]
- 131. Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules. 2015; 20: 185205. [PubMed] https://doi.org/10.3390/molecules20010185.
- 132. Gallic acid: molecular rival of cancer. Environ Toxicol Pharmacol. 2013; 35: 473-485. [PubMed]
- 133. Selective inhibition of p300 HAT blocks cell cycle progression, induces cellular senescence, and inhibits the DNA damage response in melanoma cells. J Invest Dermatol. 2013; 133: 2444-2452. [PubMed] https://doi.org/10.1038/jid.2013.187.
- 134. Targeting histone deacetyalses in the treatment of B- and T-cell malignancies. Invest New Drugs. 2010; 28 Suppl 1: S58-78. [PubMed] https://doi.org/10.1007/s10637-010-9591-3.
- 135. FDA approval: Belinostat for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma. Clin Cancer Res. 2015; in press [PubMed]
- 136. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014; 15: 11951206. [PubMed]
- 137. The histone deacetylase inhibitor, romidepsin, suppresses cellular immune functions of cutaneous T-cell lymphoma patients. Am J Hematol. 2012; 87: 354-360. [PubMed] https://doi.org/10.1002/ajh.23112.
- 138. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci USA. 2000; 97: 10014-10019. [PubMed] https://doi.org/10.1073/pnas.180316197.
- 139. Genes are often sheltered from the global histone hyperacetylation induced by HDAC inhibitors. PLoS ONE. 2012; 7: e33453. [PubMed] https://doi.org/10.1371/journal.pone.0033453.
- 140. Novel mechanisms of apoptosis induced by histone deacetylase inhibitors. Cancer Res. 2003; 63: 4460-4471. [PubMed]
- 141. The expression of a small fraction of cellular genes is changed in response to histone hyperacetylation. Gene Expr. 1996; 5: 245-253. [PubMed]
- 142. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006; 5: 769-784. [PubMed]
- 143. The biology of HDAC in cancer: the nuclear and epigenetic components. Handb Exp Pharmacol. 2011; 206: 13-37. [PubMed]
- 144. Regulation of p53 responses by post-translational modifications. Cell Death Differ. 2003; 10: 400-403. [PubMed]
- 145. Bim upregulation by histone deacetylase inhibitors mediates interactions with the Bcl-2 antagonist ABT-737: evidence for distinct roles for Bcl-2, Bcl-xL, and Mcl-1. Mol Cell Biol. 2009; 29: 61496169. [PubMed] https://doi.org/10.1128/MCB.01481-08.
- 146. The sensitivity of diffuse large B-cell lymphoma cell lines to histone deacetylase inhibitor-induced apoptosis is modulated by BCL-2 family protein activity. PLoS ONE. 2013; 8: e62822. [PubMed] https://doi.org/10.1371/journal.pone.0062822.
- 147. Mechanisms of resistance to histone deacetylase inhibitors. Adv Cancer Res. 2012; 116: 39-86. [PubMed]
- 148. Biomarkers for predicting clinical responses to HDAC inhibitors. Cancer Lett. 2009; 280: 177-183. [PubMed]
- 149. HR23B is a biomarker for tumor sensitivity to HDAC inhibitor-based therapy. Proc Natl Acad Sci USA. 2010; 107: 6532-6537. [PubMed] https://doi.org/10.1073/pnas.0913912107.
- 150. Histone deacetylase inhibitors: advancing therapeutic strategies in hematological and solid malignancies. Pharmaceuticals (Basel). 2010; 3: 2411-2469. [PubMed] https://doi.org/10.3390/ph3082441.
- 151. Novel HDAC inhibitors exhibit pre-clinical efficacy in lymphoma models and point to the importance of CDKN1A expression levels in mediating their anti-tumor response. Oncotarget. 2015; 6: 5059-5071. [PubMed] https://doi.org/10.18632/oncotarget.3239.
- 152. (2014). FDA grants orphan drug designation to Mocetinostat for diffuse large B-cell lymphoma. Retrieved from www.ascopost.com/ViewNews.aspx?nid=17491 [PubMed] https://doi.org/10.1371/journal.pone.0216832.
- 153. (2014). Mirati Therapeutics Receives Orphan Drug Designation from U.S. Food & Drug Administration for Mocetinostat in Myelodysplastic Syndrome. [PubMed] https://doi.org/10.1371/journal.pone.0216832.
- 154. Somatic mutations of the CREBBP and EP300 genes affect response to histone deacetylase inhibition in malignant DLBCL clones. Leuk Res Rep. 2012; 2: 1-3. [PubMed] https://doi.org/10.1016/j.lrr.2012.10.002.
- 155. Acetylation and deacetylation regulate CCAAT/enhancer binding protein beta at K39 in mediating gene transcription. Mol Cell Endocrinol. 2008; 289: 94-101. [PubMed]
- 156. GCN5 acetylates and regulates the stability of the oncoprotein E2A-PBX1 in acute lymphoblastic leukemia. Leukemia. 2013; 27: 578-585. [PubMed]
- 157. Mapping acetylation sites in E2A identifies a conserved lysine residue in activation domain 1 that promotes CBP/p300 recruitment and transcriptional activation. Biochim Biophys Acta. 2012; 1819: 375-381. [PubMed]
- 158. Functional regulation of GATA-2 by acetylation. J Leukoc Biol. 2004; 75: 529-540. [PubMed]
- 159. Interferon regulatory factor-2 regulates cell growth through its acetylation. J Biol Chem. 2003; 278: 2540125407. [PubMed]
- 160. A specific lysine in c-Jun is required for transcriptional repression by E1A and is acetylated by p300. EMBO J. 2001; 20: 6095-6103. [PubMed] https://doi.org/10.1093/emboj/20.21.6095.
- 161. Increased affinity of c-Myb for CREB-binding protein (CBP) after CBP-induced acetylation. J Biol Chem. 2001; 276: 3674-3682. [PubMed]
- 162. The ins and outs of MYC regulation by posttranslational mechanisms. J Biol Chem. 2006; 281: 34725-34729. [PubMed]
- 163. Extracellular signal-regulated kinase 1/2-mediated phosphorylation of p300 enhances myosin heavy chain I/β gene gene expression via acetylation of nuclear factor of activated T cells c1. Nucleic Acids Res. 2011; 39: 5907-5925. [PubMed] https://doi.org/10.1093/nar/gkr162.
- 164. Acetylation-dependent regulation of endothelial Notch signalling by the SIRT1 deacetylase. Nature. 2011; 473: 234-238. [PubMed] https://doi.org/10.1038/nature09917.
- 165. Acetylation controls Notch3 stability and function in T-cell leukemia. Oncogene. 2012; 31: 3807-3817. [PubMed]
- 166. Functional analysis of the roles of posttranslational modifications at the p53 C terminus in regulating p53 stability and activity. Mol Cell Biol. 2005; 25: 5389-5395. [PubMed] https://doi.org/10.1128/MCB.25.13.5389-5395.2005.
- 167. Histone acetyltransferase p300 acetylates Pax5 and strongly enhances Pax5-mediated transcriptional activity. J Biol Chem. 2011; 286: 1413714145. [PubMed] https://doi.org/10.1074/jbc.M110.176289.
- 168. Protein acetylation regulates both PU.1 transactivation and and Igκ 3’ enhancer activity. J Immunol. 2005; 175: 5160-5169. [PubMed]
- 169. Posttranslational modifications of RUNX1 as potential anticancer targets. Oncogene. 2014; 0. [PubMed]
- 170. FGF2-activated ERK mitogen-activated protein kinase enhances Runx2 acetylation and stabilization. J Biol Chem. 2010; 285: 3568-3574. [PubMed] https://doi.org/10.1074/jbc.M109.055053.
- 171. Phosphorylation, acetylation and ubiquitination: the molecular basis of RUNX regulation. Gene. 2006; 366: 58-66. [PubMed]
- 172. Smad3 is acetylated by p300/CBP to regulate its transactivation activity. Oncogene. 2007; 26: 500-508. [PubMed]
- 173. Post-translational control of Sp-family transcription factors. Curr Genomics. 2008; 9: 301-311. [PubMed] https://doi.org/10.2174/138920208785133244.
- 174. STAT5 acetylation: Mechanisms and consequences for immunological control and leukemogenesis. JAKSTAT. 2013; 2: e26102. [PubMed] https://doi.org/10.4161/jkst.26102.
- 175. Acetylation of human TCF4 (TCF7L2) proteins attenuates inhibition by the HBP1 repressor and induces a conformational change in the TCF4::DNA complex. PLoS ONE. 2013; 8: e61867. [PubMed] https://doi.org/10.1371/journal.pone.0061867.
- 176. Regulation of transcription factor YY1 by acetylation and deacetylation. Mol Cell Biol. 2001; 21: 5979-5991. [PubMed] https://doi.org/10.1128/MCB.21.17.5979-5991.2001.
- 177. SIRT1 links CIITA deacetylation to MHC II activation. Nucleic Acids Res. 2011; 39: 9549-9558. [PubMed] https://doi.org/10.1093/nar/gkr651.
- 178. Acetylation of Ets-1 is the key to chromatin remodeling for miR-192 expression. Sci Signal. 2013; 6: pe21. [PubMed]
- 179. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2012; 12: 9-22. [PubMed] https://doi.org/10.1038/nrc3183.
- 180. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc Natl Acad Sci USA. 2012; 109: 3879-3884. [PubMed] https://doi.org/10.1073/pnas.1121343109.
- 181. Mutational and structural analysis of diffuse large B-cell lymphoma using wholegenome sequencing. Blood. 2013; 122: 1256-1265. [PubMed] https://doi.org/10.1182/blood-2013-02-483727.
- 182. CREBBP mutations in relapsed acute lymphoblastic leukaemia. Nature. 2011; 471: 235-239. [PubMed] https://doi.org/10.1038/nature09727.
- 183. Histone deacetylase in chronic lymphocytic leukemia. Oncology. 2011; 81: 325-329. [PubMed]
- 184. Regulation of STAT3 by histone deacetylase-3 in diffuse large B-cell lymphoma: implications for therapy. Leukemia. 2012; 26: 1356-1364. [PubMed] https://doi.org/10.1038/leu.2011.340.
- 185. Histone deacetylase inhibitor potentiates chemotherapy-induced apoptosis through Bim upregulation in Burkitt’s lymphoma cells. J Cancer Res Clin Oncol. 2012; 138: 317-325. [PubMed]
- 186. Trichostatin A, sodium butyrate, and 5-aza-2’-deoxycytidine alter the expression of glucocorticoid receptor α and β isoforms in Hut-78 T- and Raji B-lymphoma cell lines. Biomed Pharmacother. 2007; 61: 451-454. [PubMed]
- 187. Peroxisome proliferator-activated receptor gamma agonists potentiate the cytotoxic effect of valproic acid in multiple myeloma cells. Br J Haematol. 2009; 147: 662-671. [PubMed]
- 188. HDAC inhibitors potentiate the apoptotic effect of enzastaurin in lymphoma cells. Apoptosis. 2011; 16: 914923. [PubMed]
- 189. Synergistic interactions between HDAC and sirtuin inhibitors in human leukemia cells. PLoS ONE. 2011; 6: e22739. [PubMed] https://doi.org/10.1371/journal.pone.0022739.
- 190. Valproic acid as epigenetic cancer drug: preclinical, clinical and transcriptional effects on solid tumors. Cancer Treat Rev. 2008; 34: 206-222. [PubMed]
- 191. Antitumor activities of valproic acid on Epstein-Barr virus-associated T and natural killer lymphoma cells. Cancer Sci. 2012; 103: 375-381. [PubMed]
- 192. Valproic acid for the treatment of myeloid malignancies. Cancer. 2007; 110: 943-954. [PubMed]
- 193. Synergistic/additive interaction of valproic acid with bortezomib on proliferation and apoptosis of acute myeloid leukemia cells. Leuk Lymphoma. 2012; 53: 2487-2495. [PubMed]
- 194. Histone deacetylase inhibitors down-regulate bcl-2 expression and induce apoptosis in t(14;18) lymphomas. Mol Cell Biol. 2005; 25: 1608-1619. [PubMed] https://doi.org/10.1128/MCB.25.5.1608-1619.2005.
- 195. Involvement of caspase activation and mitochondrial stress in trichostatin A-induced apoptosis of Burkitt’s lymphoma cell line, Akata. J Cell Biochem. 2006; 99: 1420-1430. [PubMed]
- 196. Anticancer activities of trichostatin A on maligant lymphoid cells. J Huazhong Univ Sci Technolog Med Sci. 2006; 26: 538-541. [PubMed]
- 197. Induction of ID1 expression and apoptosis by the histone deacetylase inhibitor (trichostatin A) in human acute myeloid leukaemic cells. Cell Prolif. 2008; 41: 86-97. [PubMed]
- 198. Histone deacetylation : an attractive target for cancer therapy? Drugs R D. 2008; 9: 369-383.. 2008; 41: 86-97. [PubMed]
- 199. Vorinostat inhibits STAT6-mediated TH2 cytokine and TARC production and induces cell death in Hodgkin lymphoma cell lines. Blood. 2008; 112: 14241433. [PubMed] https://doi.org/10.1182/blood-2008-01-133769.
- 200. Phase II trial of oral vorinostat (suberoylanilide hydroxamic acid) in relapsed diffuse large-B-cell lymphoma. Ann Oncol. 2008; 19: 964-969. [PubMed]
- 201. The pan-HDAC inhibitor vorinostat potentiates the activity of the proteasome inhibitor carfilzomib in human DLBCL cells in vitro and in vivo. Blood. 2010; 115: 4478-4487. [PubMed] https://doi.org/10.1182/blood-2009-12-257261.
- 202. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007; 5: 981-989. [PubMed]
- 203. Enhancing the apoptotic and therapeutic effects of HDAC inhibitors. Cancer Lett. 2009; 280: 125-133. [PubMed]
- 204. Vorinostat for treatment of cutaneous manifestations of advanced primary cutaneous T-cell lymphoma. Clin Cancer Res. 2007; 13: 2318-2322. [PubMed]
- 205. Dimethyl sulfoxide to vorinostat: development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol. 2007; 25: 84-90. [PubMed]
- 206. Romidepsin and belinostat synergize the antineoplastic effect of bortezomib in mantle cell lymphoma. Clin Cancer Res. 2010; 16: 554-565. [PubMed]
- 207. Vorinostat and bortezomib exert synergistic antiproliferative and proapoptotic effects in colon cancer cell models. Mol Cancer Ther. 2009; 8: 342-349. [PubMed] https://doi.org/10.1158/1535-7163.MCT-08-0534.
- 208. Defining the target specificity of ABT-737 and synergistic antitumor activities in combination with histone deacetylase inhibitors. Blood. 2009; 113: 19821991. [PubMed]
- 209. Deciphering the molecular events necessary for synergistic tumor cell apoptosis mediated by the histone deacetylase inhibitor vorinostat and the BH3 mimetic ABT737. Cancer Res. 2011; 71: 3603-3615. [PubMed]
- 210. Interactions between bortezomib and romidepsin and belinostat in chronic lymphocytic leukemia cells. Clin Cancer Res. 2008; 14: 549-558. [PubMed]
- 211. The histone deacetylase inhibitor, PXD101, potentiates bortezomib-induced anti-multiple myeloma effect by induction of oxidative stress and DNA damage. Br J Haematol. 2007; 139: 385-397. [PubMed]
- 212. Belinostat: a new broad acting antineoplastic histone deacetylase inhibitor. Expert Opin Investig Drugs. 2009; 18: 501-508. [PubMed]
- 213. A phase I clinical trial of the histone deacetylase inhibitor belinostat in patients with advanced hematological neoplasia. Eur J Haematol. 2008; 81: 170-176. [PubMed]
- 214. The preclinical activity of the histone deacetylase inhibitor PXD101 (belinostat) in hepatocellular carcinoma cell lines. Invest New Drugs. 2010; 28: 107-114. [PubMed]
- 215. Restoring the functional immunogenicity of chronic lymphocytic leukemia using epigenetic modifiers. Leuk Res. 2011; 35: 394-404. [PubMed] https://doi.org/10.1016/j.leukres.2010.08.001.
- 216. Cotreatment with histone deacetylase inhibitor LAQ824 enhances Apo-2L/tumor necrosis factor-related apoptosis inducing ligand-induced death inducing signaling complex activity and apoptosis of human acute leukemia cells. Cancer Res. 2004; 64: 25802589. [PubMed]
- 217. Use of a novel histone deacetylase inhibitor to induce apoptosis in cell lines of acute lymphoblastic leukemia. Haematologica. 2004; 89: 419-426. [PubMed]
- 218. The deacetylase inhibitor LAQ824 induces notch signalling in haematopoietic progenitor cells. Leuk Res. 2011; 35: 119125. [PubMed]
- 219. Histone deacetylase inhibitor NVP-LAQ824 has significant activity against myeloid leukemia cells in vitro and in vivo. Leukemia. 2004; 18: 1951-1963. [PubMed]
- 220. Preliminary evidence of disease response to the pan deacetylase inhibitor panobinostat (LBH589) in refractory Hodgkin Lymphoma. Br J Haematol. 2009; 147: 97-101. [PubMed]
- 221. Histone deacetylase inhibitor panobinostat induces clinical responses with associated alterations in gene expression profiles in cutaneous T-cell lymphoma. Clin Cancer Res. 2008; 14: 4500-4510. [PubMed]
- 222. A phase I study of intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res. 2006; 12: 4628-4635. [PubMed]
- 223. Histone deacetylases and the immunological network: implications in cancer and inflammation. Oncogene. 2010; 29: 157-173. [PubMed]
- 224. Effective targeting of quiescent chronic myelogenous leukemia stem cells by histone deacetylase inhibitors in combination with imatinib mesylate. Cancer Cell. 2010; 17: 427-442. [PubMed] https://doi.org/10.1016/j.ccr.2010.03.011.
- 225. Notch1 signaling is irresponsible to the anti-leukemic effect of HDACis in B-ALL Nalm-6 cells. Ann Hematol. 2013; 92: 33-39. [PubMed]
- 226. Hyper-activation of WNT/beta-catenin signaling pathway mediates anti-tumor effects of histone deacetylase inhibitors in acute T lymphoblastic leukemia. Leuk Lymphoma. 2012; 53: 1769-1778. [PubMed]
- 227. A histone deacetylase inhibitor, azelaic bishydroxamic acid, shows cytotoxicity on Epstein-Barr virus transformed B-cell lines: a potential therapy for posttransplant lymphoproliferative disease. Transplantation. 2002; 73: 271-279. [PubMed]
- 228. Combination of SK-7041, one of novel histone deacetylase inhibitors, and STI571-induced synergistic apoptosis in chronic myeloid leukemia. Anticancer Drugs. 2007; 18: 641-647. [PubMed]
- 229. Class I histone deacetylase-selective novel synthetic inhibitors potently inhibit human tumor proliferation. Clin Cancer Res. 2004; 10: 5271-5281. [PubMed]
- 230. Tubacin suppresses proliferation and induces apoptosis of acute lymphoblastic leukemia cells. Leuk Lymphoma. 2011; 52: 1544-1555. [PubMed] https://doi.org/10.3109/10428194.2011.570821.
- 231. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc Natl Acad Sci USA. 2005; 102: 8567-8572. [PubMed] https://doi.org/10.1073/pnas.0503221102.
- 232. Tubacin kills Epstein-Barr virus (EBV)-Burkitt lymphoma cells by inducing reactive oxygen species and EBV lymphoblastoid cells by inducing apoptosis. J Biol Chem. 2009; 284: 17102-17109. [PubMed] https://doi.org/10.1074/jbc.M809090200.
- 233. HDAC6 inhibition enhances 17-AAG—mediated abrogation of hsp90 chaperone function in human leukemia cells. Blood. 2008; 112: 1886-1893. [PubMed]
- 234. Preclinical anti-myeloma activity of the novel HDAC-inhibitor JNJ-26481585. Br J Haematol. 2010; 149: 529-536. [PubMed]
- 235. PCI-24781 induces caspase and reactive oxygen species-dependent apoptosis through NF-κB mechanisms and is synergistic with bortezomib in lymphoma cells. Clin Cancer Res. 2009; 15: 3354-3365. [PubMed] https://doi.org/10.1158/1078-0432.CCR-08-2365.
- 236. PCI-24781, a novel hydroxamic acid HDAC inhibitor, exerts cytotoxicity and histone alterations via aaspase-8 and FADD in leukemia cells. Int J Cell Biol. 2010; 2010: 207420. [PubMed] https://doi.org/10.1155/2010/207420.
- 237. MS-275, a potent orally available inhibitor of histone deacetylases—the development of an anticancer agent. Int J Biochem Cell Biol. 2007; 39: 1388-1405. [PubMed]
- 238. Blockade of mTOR signaling potentiates the ability of histone deacetylase inhibitor to induce growth arrest and differentiation of acute myelogenous leukemia cells. Leukemia. 2008; 22: 2159-2168. [PubMed]
- 239. Entinostat, a novel histone deacetylase inhibitor is active in B-cell lymphoma and enhances the anti-tumour activity of rituximab and chemotherapy agents. Br J Haematol. 2015: 169: 506-519. [PubMed] https://doi.org/10.1111/bjh.13318.
- 240. The histone deacetylase inhibitor MS-275 promotes differentiation or apoptosis in human leukemia cells through a process regulated by generation of reactive oxygen species and induction of p21CIP/WAF1. Cancer Res. 2003; 63: 3637-3645. [PubMed]
- 241. TORC1 and class I HDAC inhibitors synergize to suppress mature B cell neoplasms. Mol Oncol. 2014; 8: 261-272. [PubMed] https://doi.org/10.1016/j.molonc.2013.11.007.
- 242. Evaluation of the pharmacodynamic effects of MGCD0103 from preclinical models to human using a novel HDAC enzyme assay. Clin Cancer Res. 2008; 14: 3441-3449. [PubMed] https://doi.org/10.1158/1078-0432.CCR-07-4427.
- 243. The class-I HDAC inhibitor MGCD0103 induces apoptosis in Hodgkin lymphoma cell lines and synergizes with proteasome inhibitors by an HDAC6-independent mechanism. Br J Haematol. 2010; 151: 387-396. [PubMed] https://doi.org/10.1111/j.1365-2141.2010.08342.x.
- 244. The histone deacetylase inhibitor MGCD0103 induces apoptosis in B-cell chronic lymphocytic leukemia cells through a mitochondria-mediated caspase activation cascade. Mol Cancer Ther. 2010; 9: 1349-1360. [PubMed]
- 245. MGCD0103, a novel isotypeselective histone deacetylase inhibitor, has broad spectrum antitumor activity in vitro and in vivo. Mol Cancer Ther. 2008; 7: 759-768. [PubMed]
- 246. Phase 1 study of the oral isotype specific histone deacetylase inhibitor MGCD0103 in leukemia. Blood. 2008; 112: 981-989. [PubMed] https://doi.org/10.1182/blood-2007-10-115873.
- 247. Promising antitumor activity with MGCD0103, a novel isotype-selective histone deacetylase inhibitor. Expert Opin Investig Drugs. 2008; 17: 1247-1254. [PubMed]
- 248. The anti-leukemic effect and molecular mechanisms of novel hydroxamate and benzamide histone deacetylase inhibitors with 5-aza-cytidine. Int J Oncol. 2011; 38: 14211425. [PubMed]
- 249. The combination of a histone deacetylase inhibitor with the BH3-mimetic GX15-070 has synergistic antileukemia activity by activating both apoptosis and autophagy. Autophagy. 2010; 6: 976-978. [PubMed]
- 250. Mocetinostat for relapsed classical Hodgkin’s lymphoma: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2011; 12: 1222-1228. [PubMed] https://doi.org/10.1016/S1470-2045(11)70265-0.
- 251. Romidepsin for the treatment of cutaneous T-cell lymphoma. Drugs Today (Barc). 2009; 45: 787-795. [PubMed]
- 252. FK228 (depsipeptide) as a natural prodrug that inhibits class I histone deacetylases. Cancer Res. 2002; 62: 4916-4921. [PubMed]
- 253. Depsipeptide induces cell death in Hodgkin lymphoma-derived cell lines. Leuk Res. 2009; 33: 929-936. [PubMed]
- 254. Clinically meaningful reduction in pruritus in patients with cutaneous T-cell lymphoma treated with romidepsin. Leuk Lymphoma. 2013; 54: 284289. [PubMed]
- 255. Depsipeptide (FK228) as a novel histone deacetylase inhibitor: mechanism of action and anticancer activity. Mini Rev Med Chem. 2007; 7: 1062-1069. [PubMed]
- 256. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood. 2011; 117: 5827-5834. [PubMed] https://doi.org/10.1182/blood-2010-10-312603.
- 257. Phase II multiinstitutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2009; 27: 5410-5417. [PubMed] https://doi.org/10.1200/JCO.2008.21.6150.
- 258. Inhibitor of histone deacetylation, depsipeptide (FR901228), in the treatment of peripheral and cutaneous T-cell lymphoma: a case report. Blood. 2001; 98: 28652868. [PubMed]
- 259. HDAC inhibitors for the treatment of cutaneous T-cell lymphomas. Future Med Chem. 2012; 4: 471-486. [PubMed]
- 260. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol. 2010; 28: 4485-4491. [PubMed]
- 261. c-Jun blocks cell differentiation but not growth inhibition or apoptosis of chronic myelogenous leukemia cells induced by STI571 and by histone deacetylase inhibitors. J Cell Physiol. 2009; 218: 568-574. [PubMed]
- 262. Apicidin potentiates the imatinib-induced apoptosis of Bcr-Abl-positive human leukaemia cells by enhancing the activation of mitochondria-dependent caspase cascades. Br J Haematol. 2004; 124: 166-178. [PubMed]
- 263. Apicidin, a histone deacetylase inhibitor, induces apoptosis and Fas/Fas ligand expression in human acute promyelocytic leukemia cells. J Biol Chem. 2002; 277: 2073-2080. [PubMed]
- 264. Cotreatment with apicidin overcomes TRAIL resistance via inhibition of Bcr-Abl signaling pathway in K562 leukemia cells. Exp Cell Res. 2009; 315: 1809-1818. [PubMed]
- 265. Nicotinamide blocks proliferation and induces apoptosis of chronic lymphocytic leukemia cells through activation of the p53/miR-34a/SIRT1 tumor suppressor network. Cancer Res. 2011; 71: 4473-4483. [PubMed]
- 266. Activation of p53 by SIRT1 inhibition enhances elimination of CML leukemia stem cells in combination with imatinib. Cancer Cell. 2012; 21: 266-281. [PubMed] https://doi.org/10.1016/j.ccr.2011.12.020.
- 267. Amurensin G, a novel SIRT1 inhibitor, sensitizes TRAIL-resistant human leukemic K562 cells to TRAIL-induced apoptosis. Biochem Pharmacol. 2012; 84: 402-410. [PubMed]
- 268. Clinical experience with intravenous and oral formulations of the novel histone deacetylase inhibitor suberoylanilide hydroxamic acid in patients with advanced hematologic malignancies. J Clin Oncol. 2006; 24: 166173. [PubMed]
- 269. Potential efficacy of the oral histone deacetylase inhibitor vorinostat in a phase I trial in follicular and mantle cell lymphoma. Cancer Sci. 2010; 101: 196-200. [PubMed]
- 270. A phase I study of vorinostat in combination with idarubicin in relapsed or refractory leukaemia. Br J Haematol. 2010; 150: 72-82. [PubMed] https://doi.org/10.1111/j.1365-2141.2010.08211.x.
- 271. Phase I trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) in patients with advanced multiple myeloma. Leuk Lymphoma. 2008; 49: 502-507. [PubMed]
- 272. Phase I study of vorinostat in combination with bortezomib for relapsed and refractory multiple myeloma. Clin Cancer Res. 2009; 15: 5250-5257. [PubMed] https://doi.org/10.1158/1078-0432.CCR-08-2850.
- 273. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood. 2008; 111: 1060-1066. [PubMed]
- 274. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005; 23: 3923-3931. [PubMed] https://doi.org/10.1093/annonc/mdv066.
- 275. Phase II trial of vorinostat with idarubicin and cytarabine for patients with newly diagnosed acute myelogenous leukemia or myelodysplastic syndrome. J Clin Oncol. 2012; 30: 22042210. [PubMed]
- 276. Phase II trial of vorinostat and gemtuzumab ozogamicin as induction and post-remission therapy in older adults with previously untreated acute myeloid leukemia. Haematologica. 2012; 97: 739-742. [PubMed] https://doi.org/10.3324/haematol.2011.055822.
- 277. A phase 2 study of vorinostat for treatment of relapsed or refractory Hodgkin lymphoma: Southwest Oncology Group Study S0517. Leuk Lymphoma. 2012; 53: 259-262. [PubMed] https://doi.org/10.3109/10428194.2011.608448.
- 278. Phase II study of vorinostat for treatment of relapsed or refractory indolent non-Hodgkin’s lymphoma and mantle cell lymphoma. J Clin Oncol. 2011; 29: 1198-1203. [PubMed] https://doi.org/10.1200/JCO.2010.32.1398.
- 279. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood. 2007; 109: 31-39. [PubMed] https://doi.org/10.1182/blood-2006-06-025999.
- 280. Evaluation of the long-term tolerability and clinical benefit of vorinostat in patients with advanced cutaneous T-cell lymphoma. Clin Lymphoma Myeloma. 2009; 9: 412-416. [PubMed]
- 281. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007; 25: 31093115. [PubMed]
- 282. Vorinostat plus bortezomib for the treatment of relapsed/refractory multiple myeloma: a case series illustrating utility in clinical practice. Clin Lymphoma Myeloma Leuk. 2010; 10: 149151. [PubMed]
- 283. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012; 30: 631-636. [PubMed]
- 284. Low-dose electron beam radiation and romidepsin therapy for symptomatic cutaneous T-cell lymphoma lesions. Br J Dermatol. 2012; 167: 194-197. [PubMed] https://doi.org/10.1111/j.1365-2133.2012.10905.x.
- 285. A phase I study of panobinostat in combination with ICE (Ifosfamide, Carboplatin and Etoposide) in patients with relapsed or refractory classical Hodgkin lymphoma (cHL). Clin Adv Hematol Oncol. 2014; 12: 5-6. [PubMed]
- 286. Phase I study of panobinostat plus everolimus in patients with relapsed or refractory lymphoma. Clin Cancer Res. 2013; 19: 68826890. [PubMed] https://doi.org/10.1158/1078-0432.CCR-13-1906.
- 287. Phase Ib study of panobinostat and bortezomib in relapsed or relapsed and refractory multiple myeloma. J Clin Oncol. 2013; 31: 3696-3703. [PubMed]
- 288. A phase 1/2 study of oral panobinostat combined with melphalan for patients with relapsed or refractory multiple myeloma. Ann Hematol. 2014; 93: 89-98. [PubMed]
- 289. Phase II trial of the pan-deacetylase inhibitor panobinostat as a single agent in advanced relapsed/refractory multiple myeloma. Leuk Lymphoma. 2012; 53: 1820-1823. [PubMed]
- 290. PANORAMA 2: panobinostat in combination with bortezomib and dexamethasone in patients with relapsed and bortezomib-refractory myeloma. Blood. 2013; 122: 2331-2337. [PubMed]
- 291. Panobinostat activity in both bexarotene-exposed and -naive patients with refractory cutaneous T-cell lymphoma: results of a phase II trial. Eur J Cancer. 2013; 49: 386-394. [PubMed]
- 292. Panobinostat in patients with relapsed/refractory Hodgkin’s lymphoma after autologous stem-cell transplantation: results of a phase II study. J Clin Oncol. 2012; 30: 2197-2203. [PubMed]
- 293. Phase II study of melphalan, thalidomide and prednisone combined with oral panobinostat in patients with relapsed/refractory multiple myeloma. Leuk Lymphoma. 2012; 53: 1722-1727. [PubMed]
- 294. A Phase II trial of Belinostat (PXD101) in patients with relapsed or refractory peripheral or cutaneous T-cell lymphoma. Br J Haematol. 2015; 168: 811-819. [PubMed] https://doi.org/10.18632/oncotarget.26900.
Last Modified: 2016-06-04 22:32:50 EDT
PII: 65
