Genes & Cancer
CEACAMS 1, 5, and 6 in disease and cancer: interactions with pathogens
Jerin Thomas1, Addison Klebanov2, Sahara John2, Larry S. Miller3, Anil Vegesna2, Richard L. Amdur4, Krishanu Bhowmick2 and Lopa Mishra2,5
1 Donald and Barbara Zucker School of Medicine, Hempstead, NY 11549, USA
2 The Institute for Bioelectronic Medicine, The Feinstein Institutes for Medical Research, Northwell Health, Manhassett, NY 11030, USA
3 Department of Medicine, The Institute for Bioelectronic Medicine, The Feinstein Institutes for Medical Research, Division of Gastroenterology, Northwell Health, Manhassett, NY 11030, USA
4 Quantitative Intelligence Unit, The Institutes for Health Systems Science and Bioelectronic Medicine, The Feinstein Institutes for Medical Research, Northwell Health, Manhassett, NY 11030, USA
5 Cold Spring Harbor Laboratory, Cold Spring Harbor, NY 11724, USA
Correspondence to: Lopa Mishra, Larry S. Miller and Krishanu Bhowmick, email: [email protected], [email protected], [email protected]
Keywords: CEACAMs; cancer; immunology; inflammatory diseases
Received: August 24, 2022
Accepted: January 20, 2023
Published: February 01, 2023
Copyright: © 2023 Thomas et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ABSTRACT
The CEA family comprises 18 genes and 11 pseudogenes located at chromosome 19q13.2 and is divided into two main groups: cell surface anchored CEA-related cell adhesion molecules (CEACAMs) and the secreted pregnancy-specific glycoproteins (PSGs). CEACAMs are highly glycosylated cell surface anchored, intracellular, and intercellular signaling molecules with diverse functions, from cell differentiation and transformation to modulating immune responses associated with infection, inflammation, and cancer. In this review, we explore current knowledge surrounding CEACAM1, CEACAM5, and CEACAM6, highlight their pathological significance in the areas of cancer biology, immunology, and inflammatory disease, and describe the utility of murine models in exploring questions related to these proteins.
INTRODUCTION
Carcinoembryonic antigen (CEA), one of few FDA-approved biomarkers for cancer, was first identified and described in 1965 as a tumor-specific antigen expressed in embryonic gut, liver, and pancreas tissues, as well as gastrointestinal and respiratory malignancies, but not in differentiated adult tissues [1]. Like other developmental pathways and members, such as WNT, NOTCH, and Hedgehog, involved in many aspects of embryogenesis, CEA is repressed in the normal human digestive and respiratory systems but reappears during malignant transformation [1, 2]. Discovery of similar CEA-like proteins led to a nomenclature meeting reorganizing the family of proteins as carcinoembryonic antigen-related cellular adhesion molecules, or CEACAMs [3]. Notably, CEACAM1, CEACAM5, and CEACAM6 have been found to be implicated in immune related disease and cancer [4–8], and are now considered valid clinical biomarkers and promising therapeutic targets in melanoma, lung, colorectal, and pancreatic cancers. CEACAM1 is a biomarker in melanoma [9, 10], non-small cell lung cancer [11] and pancreatic adenocarcinoma (PDAC), and its increased expression is associated with severe disease. CEACAM5 has a significant clinical role as a tumor marker for several tumors including gastrointestinal and respiratory malignancies [6, 12, 13]. However, due to low sensitivity and specificity, its predictive value alone is still unclear. CEACAM6 is highly expressed in hyperplastic polyps and colon adenomas [14], breast cancer, pancreatic cancer, mucinous ovarian cancer, gastric cancer, and lung adenocarcinoma [15].
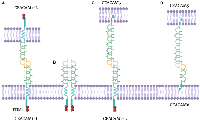
All CEACAMs contain an N-terminal V set fold from the immunoglobulin (Ig) superfamily, up to three type 2 immunoglobulins, a transmembrane domain, and a cytoplasmic domain, which facilitate adhesion through homophilic (CEACAM1, CEACAM5, and CEACAM6) and/or heterophilic (CEACAM1–CEACAM5, CEACAM5–CEACAM6, and CEACAM6–CEACAM8) interactions (Figure 1) [4]. Each CEACAM has a specific expression pattern [14, 16, 17]. Depending on the cell type, CEACAM1, CEACAM5, and CEACAM6 play pivotal roles in particular aspects of cancer biology and immunology [14, 18, 19].
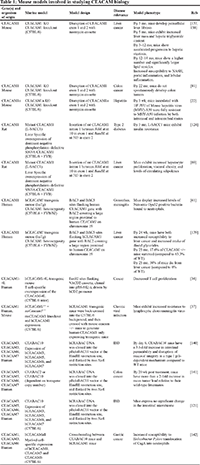
Modeling the function of CEACAMs on tumor growth, the tumor microenvironment, the immune system, and inflammatory disease has been particularly challenging, with mouse models for only a tiny number, reflecting limited expression in rodents: CEACAM1 (knockout), and collectively CEABAC transgenic pooling CEACAM3, CEACAM5, and CEACAM6 [20]. Despite this, transgenic mouse and knockout models provide substantial insight into the role of CEACAMs in disease [20–22].
FUNCTION AND EXPRESSION PATTERNS OF CEACAMS 1, 5, AND 6
CEACAM1 expression is the broadest, found in the cytoplasm of granulocytes and myeloid cells, and in the endothelium of the thyroid gland, adrenal gland, endometrium, prostate, and placenta [17]. Moreover, CEACAM1 is expressed on the cell membrane of esophageal glands, uterine glands, pancreatic ducts, prostate and cervix epithelia, liver bile ducts, enterocytes, hepatocytes, and goblet cells, with particularly strong expression in colonic absorptive cells and renal epithelia [17]. CEACAM5 is expressed similarly in the colon and pancreas but is expressed on the cell surface of gastric and intestinal mucous cells, with particularly strong staining in esophageal squamous epithelia [17]. CEACAM6 is abundantly expressed in the colon, liver, stomach, gallbladder, skin, and tongue [17]. In addition, it is expressed in the squamous epithelia of the tongue, esophagus and cervix. CEACAM6 is also prominent in the mucous epithelia of the submandibular salivary and the anterior lingual gland, as well as myeloid cells in the prostate gland and bone marrow [14].
CEACAM1 comprises several isoforms through alternative splicing, yet the functions of each of these isoforms remain to be fully elucidated. CEACAM1 isoforms differ in the amount of extracellular immunoglobulin-like domains and the length of the cytoplasmic tail. The short tail isoform (CEACAM1-S) does not have any immunoreceptor tyrosine-based inhibitory motifs (ITIMs), but contains sequences that can bind to calmodulin [23], tropomyosin, and F-actin [24]. The interaction between calmodulin and CEACAM1 results in the interference of CEACAM1 cis-homodimerization, suggesting that calmodulin regulates the activity of CEACAM1 [23]. In addition, co-sedimentation assays reveal that a glutathione S-transferase fusion protein containing the S-isoform fusion protein (GST-Cyto-S) binds to F-actin and tropomyosin, especially when incubated with G-actin during polymerization [24]. The long tail variant (CEACAM1-L) has two ITIMs that negatively regulate signaling from various activating receptors, including the T cell antigen receptor (TCR) [25]. Notably, the ratio of CEACAM1-L to CEACAM1-S varies by cell type, activation state, and growth phase [4, 26, 27].
CEACAM1 has several binding targets: intracellularly, it forms cis-dimers, which are essential in cytoplasmic signaling. In B cells, the dimeric state of CEACAM1 regulates recruitment of signaling molecules, such as the Src-family kinases and the Src homology 2 (SH2)-domain-containing protein tyrosine phosphatases (SHP1 & 2), by the cytoplasmic tail to the B cell receptor [28]. Upon phosphorylation, ITIMs on the CEACAM1-L isoform cytoplasmic tail bind to SHP1 and SHP2 and attach to B cell receptors (BCR), inhibiting BCR-induced Ca2+ mobilization [28]. CEACAM1 initially undergoes trans-homophilic (CEACAM1 - CEACAM1), or trans-heterophilic (CEACAM - CEA Family Member) dimerization, and can bind to other proteins and microbes [4, 29]. For example, trans-homophilic dimerization of CEACAM1 can protect monocytes from apoptosis through the activation of the Bcl2 protein via the phosphatidylinositol 3-kinase (PI3K)/Akt pathway [30]. CEACAM1 is involved in a variety of processes, including angiogenesis [31, 32], metabolic regulation [33], and immune modulation [27, 34–41]. In angiogenesis, CEACAM1 stimulates the proliferation, chemotaxis, and capillary-like tube formation of human microvascular endothelial cells [31], prompting further exploration into the angiogenic properties of CEACAM1 as treatment towards vascular diseases, such as atherosclerosis. Furthermore, CEACAM1 is expressed in the capillaries of numerous solid human tumors such as bladder and prostate cancer, leydig cell tumors, seminomas, and brain hemangioblastoma [31].
In immune modulation, CEACAM1 is strongly upregulated in T cells following activation by cytokines. After mitogen stimulation, CEACAM1 is rapidly mobilized in T cells from an intracellular compartment to the cell surface [42]. Based on its kinetics in T cells and inhibitory role in B cells, CEACAM1 likely plays an inhibitory role in T cell immune function. In metabolic regulation, deletion or inactivation of CEACAM1 impairs insulin clearance in mice, compromising metabolic homeostasis and promoting obesity, hepatic steatosis, and fibrosis [33]. Its signaling is complex and depends on the type and stage of tissues involved, with growth-suppressive roles in certain types of tissue but proliferative and stimulative roles in other tissue types [18]. For example, reinsertion of CEACAM1 isoforms in colorectal and prostate CEACAM1-negative tumor cells inhibits xenograft tumor development in syngeneic mice, suggesting that CEACAM1-L behaves as a tumor suppressor protein; on the contrary, CEACAM1-L overexpression in tumors correlates with metastatic spread in other types of aggressive cancers, such as hepatocellular cancer [43], melanoma, non-small cell lung, gastric, thyroid, and bladder cancers [19].
CEACAM5 (also known as CEA) comprises one N-terminal variable domain and six C2-like Ig domains [44]; its glycosylphosphatidylinositol (GPI) linker provides membrane anchoring (Figure 2) [19, 45]. CEACAM5 normally functions as an adhesion molecule [46], but it also has roles in regulating differentiation [47], immune modulation [48, 49], and inhibiting anoikis [50]. In the rat L6 myoblast cell line (a well-characterized differentiating system), stable CEA overexpression leads to fusion into myotubes, forming multinucleated myotubes. Moreover, the entire molecular program of differentiation, including creatine phosphokinase upregulation, myogenin upregulation, and β-actin downregulation, is completely abrogated by the ectopic expression of CEACAM5, suggesting that the expression of CEACAM5 inhibits terminal differentiation [47]. Furthermore, L6 rat myoblast cells transfected with CEACAM5 and CEACAM6 undergo significantly less anoikis than L6 myoblasts expressing CEACAM1 [50]. CEACAM5 is an FDA-approved diagnostic tumor marker for colon cancer, with potential as a prognostic marker [51].
CEACAM6 has an N-terminal variable domain followed by two C2-like Ig domains and is also linked to the membrane by a GPI linker (Figure 2) [45]. Like CEACAM5, CEACAM6 was shown to be a regulator of anoikis through a mechanism involving Src and focal adhesion kinase (FAK) [52]. CEACAM6 activates the Src-FAK signaling system in a dose-dependent manner, which leads to phosphorylation of MAPK/extracellular signal-regulated kinase 1/2 (MEK1/2) and extracellular signal-regulated kinase (ERK). However, there was no observed effect on Src-FAK signaling in cells treated in the same manner with CEACAM5, suggesting that CEACAM6, not CEACAM5, forms trans-dimers and activates the Src-FAK pathway. Anoikis is significantly reduced in lung cancer cells with increased expression of CEACAM6, indicating that CEACAM6 plays an essential role in inhibiting anoikis through the activation of the Src-FAK signaling system [52].
INFLAMMATORY BOWEL DISEASE
The CEACAM family of genes plays a significant role in immune modulation. A key area of study is the role of CEACAMs in binding to pathogens and their importance in inflammation. CEACAM1 is a member of the CEACAM family that is expressed in immune cells and can directly regulate immune cell activity [4, 53]. It is the only family member expressed on activated T cells [18], and it is involved in inflammatory bowel disease (IBD). Decreased expression levels of CEACAM1 are observed in pediatric Crohn’s disease (CD); however, this decrease is not found in ulcerative colitis (UC) or adult patients [7, 54]. Inhibition of CEACAM1 leads to a hyperinflammatory state that induces progression and worsens the presentation of colitis in murine models, nullifying the normal critical immunosuppressive effect of CEACAM1 [36, 39, 41, 55]. CEACAM1 expression also decreases intestinal permeability and increases epithelial barrier function in a murine model of colitis induced by dextran sulfate sodium (DSS), decreasing severity and symptomatology of the disease [56]. The increased expression of CEACAM1 in this model leads to decreased levels of proinflammatory cytokines such as TNFα and IL-6 and increased expression of tight junction proteins such as claudin1 and occludin [56]. The data suggest a protective role of CEACAM1 in models of colitis by modulating tissue and the immune environment.
CEACAM5 expression is increased in adult UC, decreased in pediatric patients, but not altered in adult CD [7]. CEACAM5 is involved in the activation of CD8+ suppressor T cells [57]. Normally, intestinal epithelial cells can promote CD8+ suppressor T cell activation by presenting soluble antigens to T cells, limiting inflammation. In patients with IBD, intestinal epithelial cells are unable to activate CD8+ suppressor T cells, leading to a proinflammatory state mediated by dysregulated cytokine release by CD4+ Th cells [58]. CEACAM6 expression is increased in pediatric CD and adult UC and CD. Compared to the role of its family members in IBD, CEACAM6 is more known for its function as a receptor for adherent invasive E. coli (AIEC) that contribute to the inflammation observed in IBD [59]. CEACAM6 expression is increased in a proinflammatory environment, and binding to AIEC LF82 would allow for continued inflammation and an increase in the availability of receptors that permit AIEC LF82 colonization, invasion, and the potential to alter the microbiome as this bacteria outcompetes other species [59, 60]. However, it is to be noted that other strains of AIEC do not induce the CEACAM6 expression [7, 59].
INTERACTIONS WITH PATHOGENS
CEACAM1 is expressed on T cells following activation, and binding to extracellular ligands or pathogens can lead to T cell downregulation [42]. Several pathogens bind to CEACAMs, including several species of Neisseria [61, 62], Haemophilus influenzae [62, 63], Helicobacter pylori [64], Moraxella catarrhalis [65], Fusobacterium nucleatum [66], Escherichia coli [59, 62, 67, 68], and species of Salmonella [68]. A mechanism of immune suppression by Neisseria involves binding their opacity-associated proteins (Opa) to CEACAM1 on immune cells and inhibiting their function without being phagocytosed [38].
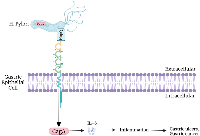
Potentially, other pathogens binding to CEACAMs exhibit similar effects [38]. Helicobactor pylori express HopQ (a surface exposed adhesin), which allows it to bind to CEACAMs, including CEACAM1, 3, 5 and 6 [69]. This binding allows an avenue of insertion for the CagA virulence factor into cells which then increases the ability of this bacteria to colonize gastric tissue and exacerbate pathologies associated with Helicobactor pylori (Figure 3) [64, 69]. H. pylori exploit human CEACAMs, using them to gain access to the cell and inject CagA into gastric epithelial cells via bacterial type IV secretion system (T4SS) [70]. After injection, CagA promotes neoplastic transformation by interfering with intracellular signaling. CagA phosphorylation and translocation into host cells is observed in CEACAM-humanized murine neutrophils, but not WT mouse neutrophils, suggesting a CEACAM driven role in CagA activation, phosphorylation, and neoplastic transformation [71]. H. pylori was shown not to interact with non-human CEACAMs, including mice, and strains of H. pylori that could colonize the gastric mucosa of mice lack a functional T4SS and do not enumerate the pathology observed in humans [72, 73]. CEACAM-humanized neutrophils and macrophages infected by CagA-positive strain of H. pylori significantly enhance MIP-1α production, leading to a large influx of active inflammatory cells in the lamina propria of gastric mucosa [71]. Infection with H. pylori is a risk factor for developing gastric ulcers and gastric cancer (Figure 3) [74]. The H. pylori cytotoxin-associated gene (CagA) is the most potent risk factor for gastric cancer [70, 74]. Blocking H. pylori from binding CEACAM1 may prevent CagA from disrupting cell signaling and inhibiting gastric ulcers and gastric cancer.
Streptococcus agalactiae, also known as group B Streptococcus (GBS) expresses β-IgI3, a newly discovered immunoglobulin fold that binds to CEACAM1 through its small α-helix and loop. In contrast, HopQ uses an intrinsically disordered loop that folds into a β hairpin and a small helix upon binding to CEACAM1 [75]. Although they bind to CEACAM1 in a structurally different manner, β-Ig13 and HopQ target the same set of CEACAM1 residues [75]. Blocking those shared residues might prove critical in preventing bacterial accumulation. Novel β-IgI3, or similar sequences, was identified in 296 proteins expressed by human gram-positive bacteria and pathogens, including S. oralis, S. pyogenes (also known as group A Streptococcus), S. dysgalactiae and S. mitis, S. intermedius, S. pneumoniae, S. pseudopneumoniae and Gemella haemolysans [75]. Exploring the role of the bacterial protein β-IgI3 in adhesion to host epithelium through binding to CEACAM1 may provide further insight into the role of CEACAMs as a docking target for both commensal and pathogenic bacteria.
Following enterotoxigenic Escherichia coli releasing heat-labile toxin in the colon, increased cyclic AMP levels are released, ultimately leading to increased CEACAM6 expression, which in turn binds to the pathogen [67]. Therefore, increased CEACAM6 may be causal in the subsequent enteropathy associated with infection of this pathogen [67]. Although immune suppression and adhesion are beneficial in some contexts, these pathogens have found ways to exploit these proteins to create a suitable environment for infection and immune evasion. Thus, disrupting the interactions between pathogens and CEACAMs is an essential avenue of interest and one that is currently being investigated [64, 74].
CANCER
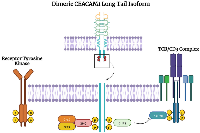
CEACAM1, CEACAM5, and CEACAM6 all have variable roles in tumor initiation, progression, and metastasis. The dual roles of CEACAM1 can be best understood through the separate functions of the extended versus short tail. ITIMs on CEACAM1-L bind to other extracellular ligands, including other CEACAM family members and CEACAM1, suppressing immune cells and allowing immune evasion by cancer cells [40, 75–78]. The cytoplasmic tail of CEACAM1 has several residues that serve as binding sites or phosphorylation sites for a variety of proteins that include: the Src family of kinases, insulin receptor, epidermal growth factor receptor upon EGF treatment, SHP1, SHP2, phorbol ester inducible staurosporine-inhibitable Ser/Thr kinases, and protein kinase C [19]. After binding to insulin, the CEACAM1 long cytoplasmic tail downregulates insulin receptor (IR) mitogenic signaling: IR phosphorylation of Tyr493 at the CEACAM1 cytoplasmic tail and recruitment of the adaptor protein Shc leads to Grb2/SOS sequestration, effectively reducing IR signaling (Figure 4) [79]. Similarly, CEACAM1 reduces epidermal growth factor receptor (EGFR) dependent cell proliferation by binding and sequestering Shc (Figure 4) [80]. The data suggest that CEACAM1 functions as a tumor suppressor in the early stages of tumorigenesis, as knockout or loss of CEACAM1 is associated with increased tumorigenic potential [81–84].
Immune cell suppression occurs after Src family kinase protein, lymphocyte-specific protein tyrosine kinase (LCK), phosphorylates the ITIMs of dimeric long tail CEACAM1, leading to recruitment of SHP2, which dephosphorylates the T cell receptor/CD3 complex and ZAP70, abrogating downstream signaling and activation of the T cell (Figure 4) [85]. CEACAM1 also downregulates natural killer cells and is involved in developing and differentiating a variety of myeloid-derived immune cells [5]. Knockout of CEACAM1 leads to the development of exhaustion-resistant and hyperinflammatory T cells, a phenotype mediated by CEACAM1-TIM-3 interactions [36]. CEACAM1 is a regulator of TIM-3, binding to TIM-3 through its N-terminal domain, forming a heterodimer, and facilitating the maturation and cell surface expression of TIM-3, which inhibits T cell activation and prevents the development of exhaustion-resistant and hyperinflammatory T cells [36]. CEACAM1 also plays a regulatory role in NK-cell-mediated cytolysis, evading NK cells by promoting intracellular retention of several NKG2D ligands [35]. Melanocytes normally do not express CEACAM1, but it was found that in melanoma cells increased expression of CEACAM1 and decreased NKG2D ligands, lead to decreased susceptibility to NK cell-mediated cytolysis [35].
Under apoptotic conditions in CT51 mouse colon carcinoma cells, CEACAM1-L is cleaved at the 457DQRD460 motif by caspase-3 within its long cytoplasmic domain, resulting in degradation of its 8 kDa cytoplasmic domain and formation of a truncated version of CEACAM1-L, the CEACAM1-LΔ461 mutant [86]. NIH 3T3 cells stably expressing mutant CEACAM1-LΔ461 show increased cell adhesion properties [86]. Cleavage of CEACAM1-L in apoptotic cells was blocked when a specific caspase-3 inhibitor was introduced, indicating CEACAM1-L is cleaved by caspase-3, but not caspase-7 or caspase-8 [86]. It remains unclear if CEACAM1-LΔ461 is tumor-promoting or tumor-suppressive. Crosslinking of CEACAM1 on the cell surface of tumor cells using monoclonal antibodies specific for CEACAM1 leads to apoptosis [84]. In a healthy colonic crypt mucosa, epithelial cells migrate towards the intestinal crypt lumen until they undergo apoptosis and shed into the gut lumen [84, 87]. Most colon tumors exhibit a marked reduction of apoptosis and decreased CEACAM1 [84]. In two cell lines of HT29 cells (colorectal adenocarcinoma cells), induction of CEA did not affect the low CEACAM1 cell line. However, it caused dose-dependent induction of apoptosis in the high CEACAM1 cell line [43], demonstrating that CEACAM5 is a mediator of CEACAM1-induced apoptosis. CEACAM1 expression is reduced in more than 85% of early colorectal adenomas and carcinomas [83]; a significant reduction is apparent in colonic aberrant crypt foci and hyperplastic polyps [83, 88]. Isolated Jurkat cells and HT29 cells displayed drastic improvement in apoptotic ability when CEACAM1 levels were high, indicating that loss of apoptosis is associated with decreased CEACAM1 expression in colorectal epithelial cells and lymphocytes [89].
Preserving the interactions between CEACAM1 and CEACAM5 might be critical in preventing colorectal adenocarcinomas. Interestingly, loss of CEACAM1 is associated with abnormal T cell function [41]. CEACAM1−/− mice exhibit significantly altered CD8+ T cell activity in the gut mucosal tissue, with CD8+ T cells in CEACAM1−/− mice expressing abundant CD29, yet less CD62L, enhancing secretion of proinflammatory cytokines IL-6, IL-17 and TNFα [41]. In addition, PD-1 and CTLA-4 are strongly upregulated on CD8+ T cells from CEACAM1−/− mice infected with Citrobacter rodentium, but neither molecule is upregulated on CD8+ T cells from infected WT mice [41]. Differences in phenotype are even stronger under colitis and are likely responsible for the higher bacterial burden and defective intestinal barrier in CEACAM1−/− mice exposed to C. rodentium induced colitis. Restoring CEACAM1 in T cells may prove critical in preventing inflammation and restoring apoptosis, as most colorectal tumors express decreased levels of CEACAM1 [83, 84].
Although its tumor suppressive properties are evident, the role of CEACAM1 in cancer is bimodal, with many similarities to the TGF-β signaling pathway; early effects being tumor suppressive, and more invasive cancers relying on it for malignant potential. For example, CEACAM1−/− mice injected with highly metastatic MC38 colorectal cancer cells and B16F10 melanoma cells experience a reduction in the number and size of metastatic lesions compared to the WT littermate, with tumor cell proliferation decreasing by 2-fold and tumor cell survival decreasing by 3-fold, indicating that expression of CEACAM1 is associated with tumorigenesis in colorectal cancer [90]. CEACAM1 interacts with TNFα, playing a pathogenic role in cirrhosis-related hyperpermeability. CEACAM1 upregulates tumor necrosis factor-α (TNFα) in a positive-feedback loop [91]. The effect is increased apoptosis, disruption of tight junction protein-maintained barrier function, and decreased restitution capacity in causing colon adenocarcinomas with hyperpermeability and cirrhosis [86, 92]. Interrupting interactions between CEACAM1 and TNFα might prove effective in preventing cirrhosis-related hyperpermeability.
In hepatocellular carcinoma (HCC), CEACAM1 cytoplasmic expression is associated with poor differentiation, high recurrence rates, larger and greater number of tumors, vascular invasion, and satellite nodules in comparison with CEACAM1 membranous expression in HCC [32]. In the liver, loss of CEACAM1 in a murine model has been found to lead to inflammation and Non-Alcoholic Steatohepatitis (NASH), with reduced insulin clearance and altered metabolism [93]. Inflammation and NASH are risk factors for developing HCC in the CEACAM1 KO mice [93]. CEACAM1 has also been shown to be a binding partner to β2-spectrin, which is involved as a cofactor for SMAD3 in the TGF-β signaling pathway [43, 94, 95]. The expression of a long tail isoform of CEACAM1 leads to the nuclear localization of SMAD3 in HCC cell lines and leads to an increased invasive phenotype [43]. Rat HCC tumor cells transfected with CEACAM1a-4L displayed a significant decrease in tumor formation, progression, and burden compared to rat HCC cells transfected with CEACAM1b-4S and the negative control group, suggesting that CEACAM1 splice-switching therapy may inhibit HCC [96].
Exploring different isoforms of CEACAM1 (CEACAM1-L, CEACAM1-S, cytoplasmic CEACAM1, and membranous CEACAM1) is critical to better understanding the variable role of CEACAM1 in colorectal cancer, gastric cancer, and HCC, and can potentially explain the contrasting results in studies exploring CEACAM1 pathology in the gastrointestinal tract. A clinical study found that CEACAM1 was expressed in the sera of 24% (15/61) of normal patients, 66% (35/53) of patients with chronic pancreatitis, and 91% (74/81) of pancreatic cancer patients, indicating that CEACAM1 may be a viable biomarker for pancreatic cancer [97, 98]. In another study, CEACAM1 was shown to be upregulated in 69% of pancreatic carcinomas compared to 2% in normal patients, and patients with low serum CEACAM1 levels have significantly increased rates of overall survival [99]. Overall, CEACAM1 is downregulated in colorectal cancer, upregulated in gastric cancer, and directly correlated with decreased overall survival in HCC and gastric cancer [32, 82–84, 97, 98].
CEACAM5 is expressed in multiple epithelial malignancies, including gastric cancer, colorectal cancer, and pancreatic cancer, as well as in NSC lung cancer and melanoma [12, 100, 101]. In coordination with CEACAM1s ability to downregulate immune cells, CEACAM5 is a binding partner to CEACAM1 that can elicit this signaling, allowing for immune evasion by cancer cells [102]. Therapies targeting CEACAM5 in cancer are being developed to block this evading immune function [103, 104]. CEACAM5 has also been shown to be important in metastasis in colorectal cancer. In colorectal cancer, it was found to prevent anoikis by binding to DR5, leading to decreased activation of caspase 8 [105]. CEACAM5 also modulates the environment in the liver to create a space on the sinusoidal endothelial cells suitable for the adhesion and survival of metastatic cells by upregulating cytokine release and increasing protection against reactive oxygen species [106].
The TGF-β pathway plays an integral role in the suppression of early colorectal cancers, yet in advanced colorectal cancers, it plays a prominent tumor promoting role—in some ways similar to CEACAM1 function [107]. CEACAM5 binds to TGF-β type I receptor (TBR1) with decreased expression of SMAD3 targets, indicating that CEACAM5 can directly inhibit the tumor suppressive properties of TGF-β [108]. The same model showed that targeting CEA or its expression rescued TGF-β signaling. Interestingly, the expression of CEACAM5 and 6 is upregulated by SMAD3-mediated TGF-β signaling, suggesting a link between the metastatic properties of TGF-β in cancer and CEACAMs [109]. Decreased expression of the TGF-β pathway has also been inversely correlated with the expression of CEACAMs. Reduction in expression of TGF-β pathway members (TBR2, SMAD4, SPTBN1) has been shown to alter the colonic microbiome shifting towards a prevalence of bacteria associated with colorectal cancer [110]. The expression of CEACAM5 and CEACAM6 in colon cancer cell lines is increased in the presence of proinflammatory cytokine interleukin 6, supporting an important role in inflammation in colorectal cancer [111].
Similar to CEACAM5, CEACAM6 expression is significantly increased in malignancies [14, 15, 112]. In addition, CEACAM6 is a useful prognostic tool for cancer [15, 113, 114]. In a cohort of 115 lung adenocarcinoma patients, expression of CEACAM6 was associated with a five-year disease-free survival rate of 49.1%, as opposed to 74.2% for CEACAM6-negative patients [8]. In a study of gastric cancer patients, it was noted that an increased level of CEACAM6 DNA in the peripheral blood was significantly associated with a higher stage of the disease as well as lymph node metastasis [115]. Analysis of tumor specimens of gastric carcinoma patients using immunohistochemistry revealed increased CEACAM6 protein levels were associated with a higher stage, and that high CEACAM6 protein levels were associated with a shorter recurrence free survival [113]. Similarly, in colon cancer patients, higher levels of CEACAM6 expression was associated with higher tumor stage, and a shorter recurrence free survival [114].
CEACAM6 is important for the metastatic potential of cancers [114, 116, 117]. CEACAM6 was found to inhibit anoikis in the pancreatic ductal adenocarcinoma (PDA) line MiaPaca2, and inhibition of CEACAM6 with short interfering RNA (siRNA) led to decreased metastasis in a nude mouse orthotopic xenograft model [118]. The PI3K-AKT signaling pathway is involved in cell survival and silencing of CEACAM6 in the MiaPaca2 cell line led to decreased AKT phosphorylation [118]. Cross linking of CEACAM6 in BxPC3 cells using antibodies leads to resistance to anoikis by activating Src in a caveolin-1 dependent manner, leading to activation of focal adhesion kinase which then signals downstream to activate several cell proliferation and survival pathways [119]. In pancreatic cancer cell lines PANC-1 and CFPAC-1, CEACAM6 is involved in the expression of epithelial to mesenchymal transition-associated genes ZEB1 and ZEB2, thereby increasing the metastatic potential of cells [116]. Downregulation of CEACAM6 increased E-Cadherin levels in colon cancer cell lines, suggesting that CEACAM6 is a factor directly responsible for these cells’ invasive properties [114].
MOUSE MODELS OF CEACAMS
The CEACAM1a null mice were initially used to provide insight into the role of CEACAM1 in insulin clearance [22, 96] and its role as a tumor suppressor in colon cancer [81], and more recently, in chronic viral infections [37] and immune regulation [36]. In CEACAM1 null mice, the absence of CEACAM1 led to an inability to clear insulin, leading to hyperinsulinemia followed by insulin resistance, obesity, and non-alcoholic steatohepatitis [120]. CEACAM1−/− mice, WT mice, and CEACAM1-4S transgenic mouse overexpressing the mouse 4S isoform (4S Tg mice) have diverse microbiomes, and the 4S Tg mice exhibit increased Gemella morbillorum, Lactobacillus murinus and Lactobacillus acidophilus, suggesting that alteration of CEACAM1-4S expression can change the relative populations of certain microorganisms in the gut [31]. The CEABAC mouse model expresses CEACAM5, CEACAM6, and CEACAM7 in various tissues and almost matches the expression patterns in humans. This bacterial artificial chromosome does not include CEACAM4 and 8, and the model utilizes continued expression of endogenous CEACAM1. Nonetheless, in the CEABAC mice, a high fat and high sugar diet, termed a “Western diet,” modifies the composition of the gut microbiome and alters the environment in the intestines, leading to increased susceptibility to AIEC, supporting the idea that IBD is a multifactorial disease involving environmental and genetic factors [121]. This model has also been used to describe an approach to treating colitis with CAR-Treg cells specific to CEA [48]. In this model, CAR-Treg cells targeted towards CEA were administered to CEABAC mice in which colitis was induced by chemical methods such as dextran sodium sulfate ingestion, or by administering CEA specific CAR CD4+ effector T cells that would lead to inflammation. The mice treated with CAR Treg cells had reduced colitis morbidity in both models of colitis [48]. Tissue from CEABAC mice was used to analyze the effects of compounds that impede the binding of AIEC [122]. However, the limitations of this model are not to be understated. The interaction that usually exists between human CEACAM6 and human CEACAM1 does not exist in this model, as murine CEACAM1 is expressed, which impedes the ability to study the interactions of human CEACAM6 and human CEACAM1 and their potential immune effects [40]. Of note, human CEACAMs are heavily modified post-translationally and the machinery for doing so is not fully present in a mouse, leading to a complex problem to fix [20].
Other murine models have also been developed, notably a human CEACAM5 transgenic mouse [21], a human CEACAM1 transgenic mouse [61], and a dominant negative phosphorylation defective CEACAM1 mutant that is overexpressed in the liver (L-SACC mouse) [123]. The L-SACC mice proved their utility in early studies that investigated the role of CEACAM1 in the clearance of insulin and insulin receptors and its effects on metabolism and the liver [123, 124]. Studies involving the CEACAM5 transgenic mouse focused mainly on developing therapies that targeted the CEACAM5 protein in cancer [49, 125–127]. They were also used to demonstrate the ability of a CEACAM5 antibody to recognize liver metastasis in colorectal cancer [128]. The mouse models are outlined in Table 1.
PERSPECTIVE
CEACAM1, 5, and 6 have shown their importance in gastrointestinal pathologies as they are closely involved with immune regulation, tumorigenesis, tumor suppression, and pathogen binding. Interrupting the interactions of CEACAM family members seems to be an interesting avenue of research, as it may be a useful tool in cancer therapy [106], and regulating immune activity in the context of IBD [57]. However, much work has yet to be done in regard to the role of CEACAMs in cellular signaling and their effects on microbiome regulation.
Key questions that remain unanswered are whether CEACAMs are drivers or sensors or both of the gut microbiome and their signaling pathways/axes from the gut to the liver and brain in modulating disease and cancer. Some of these can be addressed through existing mouse models such as CEACAM1. However, for the most part, CEACAM5 and 6, which are expressed in humans but not rodents, these interactions could be addressed through new recent and technological advances, such as STORM (a super-resolution light microscopy method that uses fluorescent probes to separate overlapping regions of individual molecules so that the position of each molecule can be more clearly determined, allows for single-molecule imaging and creation of three-dimensional fluorescence images of cells and tissues), and Cryo-electron microscopy (CryoEM)- a technique that flash-freezes microscopic protein samples in a single-molecule-thick layer of vitreous ice, provides new insight into the structure and assembly of macromolecules [129, 130]. Similarly, organoids self-assembled in vitro three-dimensional structures that are primarily generated from primary tissues or stem cells, mimic the complex aspects of their organ counterparts [131]. Organoids simulating colorectal cancer, pancreatic cancer, the gut microbiome, and HCC could prove to be an important next step. In addition, liver organoids, expressing hepatocytes, endothelial cells, stellate cells, cholangiocytes, mesenchymal, and Kupffer cells, have been developed to explore fibrosis, NAFLD, hepatobiliary functions, and transplant capabilities [132–136]. Combining CRISPR and organoid technology, expressing different levels of CEACAMs in liver organoids, may contribute to clarifying the effect of obesity and diet on CEACAM expression and adhesion in HCC. AlphaFold, a software that predicts the 3D structure of a protein based on its genomic sequence will likely prove vital in exploring the lesser-known CEACAMs, including CEACAM4, CEACAM8, CEACAM16, CEACAM18, CEACAM19, CEACAM20, and CEACAM21, in pathology, oncology, the immune system, and the microbiome.
Author contributions
JT, AK, SJ, and LM contributed to the conception and design of the review and drafted and revised the article. KB, LSM, AV, RLA, and LM revised and edited the article critically for presentation, interpretation, and discussion. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
CONFLICTS OF INTEREST
Authors have no conflicts of interest to declare.
FUNDING
This work was supported by NIH grants R01AA023146 (L. Mishra), R01CA236591 (L. Mishra), and U01CA230690 (L. Mishra).
- 1. Specific carcinoembryonic antigens of the human digestive system. J Exp Med. 1965; 122:467–81. https://doi.org/10.1084/jem.122.3.467. [Pubmed]
- 2. Expression of carcinoembryonic antigen and related genes in lung and gastrointestinal cancers. Int J Cancer. 1992; 52:718–25. https://doi.org/10.1002/ijc.2910520509. [Pubmed]
- 3. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp Cell Res. 1999; 252:243–49. https://doi.org/10.1006/excr.1999.4610. [Pubmed]
- 4. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol. 2006; 6:433–46. https://doi.org/10.1038/nri1864. [Pubmed]
- 5. CEACAM1 structure and function in immunity and its therapeutic implications. Semin Immunol. 2019; 42:101296. https://doi.org/10.1016/j.smim.2019.101296. [Pubmed]
- 6. CEACAM5 stimulates the progression of non-small-cell lung cancer by promoting cell proliferation and migration. J Int Med Res. 2020; 48:300060520959478. https://doi.org/10.1177/0300060520959478. [Pubmed]
- 7. Regulation of CEACAM Family Members by IBD-Associated Triggers in Intestinal Epithelial Cells, Their Correlation to Inflammation and Relevance to IBD Pathogenesis. Front Immunol. 2021; 12:655960. https://doi.org/10.3389/fimmu.2021.655960. [Pubmed]
- 8. Carcinoembryonic antigen-related cell adhesion molecules as surrogate markers for EGFR inhibitor sensitivity in human lung adenocarcinoma. Br J Cancer. 2012; 107:1745–53. https://doi.org/10.1038/bjc.2012.422. [Pubmed]
- 9. Protein expression of carcinoembryonic antigen cell adhesion molecules in benign and malignant melanocytic skin lesions. Am J Clin Pathol. 2009; 131:782–87. https://doi.org/10.1309/AJCP24KXJVBZXENS. [Pubmed]
- 10. Glycoconjugate profiling of primary melanoma and its sentinel node and distant metastases: implications for diagnosis and pathophysiology of metastases. Cancer Lett. 2007; 248:68–80. https://doi.org/10.1016/j.canlet.2006.05.020. [Pubmed]
- 11. Clinical and experimental studies regarding the expression and diagnostic value of carcinoembryonic antigen-related cell adhesion molecule 1 in non-small-cell lung cancer. BMC Cancer. 2013; 13:359. https://doi.org/10.1186/1471-2407-13-359. [Pubmed]
- 12. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal. 1991; 5:344–66. https://doi.org/10.1002/jcla.1860050510. [Pubmed]
- 13. Carcinoembryonic antigen is the preferred biomarker for in vivo colorectal cancer targeting. Br J Cancer. 2013; 108:662–67. https://doi.org/10.1038/bjc.2012.605. [Pubmed]
- 14. Carcinoembryonic antigen family members CEACAM6 and CEACAM7 are differentially expressed in normal tissues and oppositely deregulated in hyperplastic colorectal polyps and early adenomas. Am J Pathol. 2000; 156:595–605. https://doi.org/10.1016/S0002-9440(10)64764-5. [Pubmed]
- 15. Expression of CEACAM6 in resectable colorectal cancer: a factor of independent prognostic significance. J Clin Oncol. 2003; 21:3638–46. https://doi.org/10.1200/JCO.2003.55.135. [Pubmed]
- 16. Immunohistochemistry of carcino-embryonic antigen in the embryo, fetus and adult. Tumour Biol. 1988; 9:145–53. https://doi.org/10.1159/000217555. [Pubmed]
- 17. CD66a (BGP), an adhesion molecule of the carcinoembryonic antigen family, is expressed in epithelium, endothelium, and myeloid cells in a wide range of normal human tissues. J Histochem Cytochem. 1996; 44:35–41. https://doi.org/10.1177/44.1.8543780. [Pubmed]
- 18. CEACAM1 as a multi-purpose target for cancer immunotherapy. Oncoimmunology. 2017; 6:e1328336. https://doi.org/10.1080/2162402X.2017.1328336. [Pubmed]
- 19. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 2013; 32:643–71. https://doi.org/10.1007/s10555-013-9444-6. [Pubmed]
- 20. Novel mouse model for carcinoembryonic antigen-based therapy. Mol Ther. 2004; 9:775–85. https://doi.org/10.1016/j.ymthe.2004.03.009. [Pubmed]
- 21. Mice transgenic for the human carcinoembryonic antigen gene maintain its spatiotemporal expression pattern. Cancer Res. 1994; 54:4169–76. [Pubmed]
- 22. Ceacam1a-/- mice are completely resistant to infection by murine coronavirus mouse hepatitis virus A59. J Virol. 2004; 78:10156–65. https://doi.org/10.1128/JVI.78.18.10156-10165.2004. [Pubmed]
- 23. Calmodulin binds to specific sequences in the cytoplasmic domain of C-CAM and down-regulates C-CAM self-association. J Biol Chem. 1996; 271:1393–99. https://doi.org/10.1074/jbc.271.3.1393. [Pubmed]
- 24. Carcinoembryonic antigen cell adhesion molecule 1 directly associates with cytoskeleton proteins actin and tropomyosin. J Biol Chem. 2001; 276:47421–33. https://doi.org/10.1074/jbc.M109110200. [Pubmed]
- 25. SHP1 phosphatase-dependent T cell inhibition by CEACAM1 adhesion molecule isoforms. Immunity. 2006; 25:769–81. https://doi.org/10.1016/j.immuni.2006.08.026. [Pubmed]
- 26. The tumor growth-inhibiting cell adhesion molecule CEACAM1 (C-CAM) is differently expressed in proliferating and quiescent epithelial cells and regulates cell proliferation. Cancer Res. 2000; 60:1236–44. [Pubmed]
- 27. CEACAM1 is a potent regulator of B cell receptor complex-induced activation. J Leukoc Biol. 2003; 74:126–34. https://doi.org/10.1189/jlb.1202594. [Pubmed]
- 28. Biliary glycoprotein (BGPa, CD66a, CEACAM1) mediates inhibitory signals. J Leukoc Biol. 2001; 70:335–40. [Pubmed]
- 29. Evidence for regulated dimerization of cell-cell adhesion molecule (C-CAM) in epithelial cells. Biochem J. 1996; 320:847–53. https://doi.org/10.1042/bj3200847. [Pubmed]
- 30. CEACAM1 (CD66a) promotes human monocyte survival via a phosphatidylinositol 3-kinase- and AKT-dependent pathway. J Biol Chem. 2006; 281:39179–93. https://doi.org/10.1074/jbc.M608864200. [Pubmed]
- 31. CEA-related cell adhesion molecule 1: a potent angiogenic factor and a major effector of vascular endothelial growth factor. Mol Cell. 2000; 5:311–20. https://doi.org/10.1016/s1097-2765(00)80426-8. [Pubmed]
- 32. CEACAM1 cytoplastic expression is closely related to tumor angiogenesis and poorer relapse-free survival after curative resection of hepatocellular carcinoma. World J Surg. 2011; 35:2259–65. https://doi.org/10.1007/s00268-011-1119-2. [Pubmed]
- 33. CEACAM1 in Liver Injury, Metabolic and Immune Regulation. Int J Mol Sci. 2018; 19:3110. https://doi.org/10.3390/ijms19103110. [Pubmed]
- 34. The short isoform of the CEACAM1 receptor in intestinal T cells regulates mucosal immunity and homeostasis via Tfh cell induction. Immunity. 2012; 37:930–46. https://doi.org/10.1016/j.immuni.2012.07.016. [Pubmed]
- 35. CEACAM1 dampens antitumor immunity by down-regulating NKG2D ligand expression on tumor cells. J Exp Med. 2011; 208:2633–40. https://doi.org/10.1084/jem.20102575. [Pubmed]
- 36. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015; 517:386–90. https://doi.org/10.1038/nature13848. [Pubmed]
- 37. CEACAM1 promotes CD8+ T cell responses and improves control of a chronic viral infection. Nat Commun. 2018; 9:2561. https://doi.org/10.1038/s41467-018-04832-2. [Pubmed]
- 38. CEACAM1 dynamics during neisseria gonorrhoeae suppression of CD4+ T lymphocyte activation. J Immunol. 2008; 180:6827–35. https://doi.org/10.4049/jimmunol.180.10.6827. [Pubmed]
- 39. Role of CEACAM1 as a regulator of T cells. Ann N Y Acad Sci. 2006; 1072:155–75. https://doi.org/10.1196/annals.1326.004. [Pubmed]
- 40. T cell-mediated elimination of cancer cells by blocking CEACAM6-CEACAM1 interaction. Oncoimmunology. 2022; 11:2008110. https://doi.org/10.1080/2162402X.2021.2008110. [Pubmed]
- 41. CEACAM1 regulates CD8+ T cell immunity and protects from severe pathology during Citrobacter rodentium induced colitis. Gut Microbes. 2020; 11:1790–805. https://doi.org/10.1080/19490976.2020.1775464. [Pubmed]
- 42. Activation-induced expression of carcinoembryonic antigen-cell adhesion molecule 1 regulates mouse T lymphocyte function. J Immunol. 2002; 168:1028–35. https://doi.org/10.4049/jimmunol.168.3.1028. [Pubmed]
- 43. CEACAM1 long cytoplasmic domain isoform is associated with invasion and recurrence of hepatocellular carcinoma. Ann Surg Oncol. 2014 (Suppl 4); 21:S505–14. https://doi.org/10.1245/s10434-013-3460-1. [Pubmed]
- 44. Molecular cloning of a gene belonging to the carcinoembryonic antigen gene family and discussion of a domain model. Proc Natl Acad Sci U S A. 1987; 84:2965–69. https://doi.org/10.1073/pnas.84.9.2965. [Pubmed]
- 45. Carcinoembryonic antigen is anchored to membranes by covalent attachment to a glycosylphosphatidylinositol moiety: identification of the ethanolamine linkage site. Proc Natl Acad Sci U S A. 1988; 85:4648–52. https://doi.org/10.1073/pnas.85.13.4648. [Pubmed]
- 46. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. 1989; 57:327–34. https://doi.org/10.1016/0092-8674(89)90970-7. [Pubmed]
- 47. Human carcinoembryonic antigen, an intercellular adhesion molecule, blocks fusion and differentiation of rat myoblasts. J Cell Biol. 1993; 123:467–75. https://doi.org/10.1083/jcb.123.2.467. [Pubmed]
- 48. Suppression of murine colitis and its associated cancer by carcinoembryonic antigen-specific regulatory T cells. Mol Ther. 2014; 22:1018–28. https://doi.org/10.1038/mt.2014.41. [Pubmed]
- 49. Induction of cytotoxic T cells and their antitumor activity in mice transgenic for carcinoembryonic antigen. Cancer Immunol Immunother. 2000; 49:285–95. https://doi.org/10.1007/s002620000116. [Pubmed]
- 50. Human carcinoembryonic antigen functions as a general inhibitor of anoikis. Cancer Res. 2000; 60:3419–24. [Pubmed]
- 51. C-stage in colon cancer: implications of carcinoembryonic antigen biomarker in staging, prognosis, and management. J Natl Cancer Inst. 2011; 103:689–97. https://doi.org/10.1093/jnci/djr078. [Pubmed]
- 52. Overexpression of CEACAM6 activates Src-FAK signaling and inhibits anoikis, through homophilic interactions in lung adenocarcinomas. Transl Oncol. 2022; 20:101402. https://doi.org/10.1016/j.tranon.2022.101402. [Pubmed]
- 53. CEACAM1 and the regulation of mucosal inflammation. Mucosal Immunol. 2008 (Suppl 1); 1:S39–42. https://doi.org/10.1038/mi.2008.50. [Pubmed]
- 54. Defect in CEACAM family member expression in Crohn’s disease IECs is regulated by the transcription factor SOX9. Inflamm Bowel Dis. 2009; 15:1775–83. https://doi.org/10.1002/ibd.21023. [Pubmed]
- 55. Specific regulation of T helper cell 1-mediated murine colitis by CEACAM1. J Exp Med. 2004; 199:471–82. https://doi.org/10.1084/jem.20030437. [Pubmed]
- 56. Carcinoembryonic antigen related cellular adhesion molecule 1 alleviates dextran sulfate sodium-induced ulcerative colitis in mice. Life Sci. 2016; 149:120–28. https://doi.org/10.1016/j.lfs.2016.02.065. [Pubmed]
- 57. Characterizing CEACAM5 interaction with CD8α and CD1d in intestinal homeostasis. Mucosal Immunol. 2014; 7:615–24. https://doi.org/10.1038/mi.2013.80. [Pubmed]
- 58. Carcinoembryonic antigen (CEACAM) family members and Inflammatory Bowel Disease. Cytokine Growth Factor Rev. 2019; 47:21–31. https://doi.org/10.1016/j.cytogfr.2019.05.008. [Pubmed]
- 59. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J Clin Invest. 2007; 117:1566–74. https://doi.org/10.1172/JCI30504. [Pubmed]
- 60. AIEC infection triggers modification of gut microbiota composition in genetically predisposed mice, contributing to intestinal inflammation. Sci Rep. 2018; 8:12301. https://doi.org/10.1038/s41598-018-30055-y. [Pubmed]
- 61. Generation of human CEACAM1 transgenic mice and binding of Neisseria Opa protein to their neutrophils. PLoS One. 2010; 5:e10067. https://doi.org/10.1371/journal.pone.0010067. [Pubmed]
- 62. CEACAM1 recognition by bacterial pathogens is species-specific. BMC Microbiol. 2010; 10:117. https://doi.org/10.1186/1471-2180-10-117. [Pubmed]
- 63. A carcinoembryonic antigen-related cell adhesion molecule 1 homologue plays a pivotal role in nontypeable Haemophilus influenzae colonization of the chinchilla nasopharynx via the outer membrane protein P5-homologous adhesin. Infect Immun. 2008; 76:48–55. https://doi.org/10.1128/IAI.00980-07. [Pubmed]
- 64. Helicobacter pylori exploits human CEACAMs via HopQ for adherence and translocation of CagA. Nat Microbiol. 2016; 2:16188. https://doi.org/10.1038/nmicrobiol.2016.188. [Pubmed]
- 65. A novel cell-binding mechanism of Moraxella catarrhalis ubiquitous surface protein UspA: specific targeting of the N-domain of carcinoembryonic antigen-related cell adhesion molecules by UspA1. Mol Microbiol. 2003; 48:117–29. https://doi.org/10.1046/j.1365-2958.2003.03433.x. [Pubmed]
- 66. Fusobacterium spp. target human CEACAM1 via the trimeric autotransporter adhesin CbpF. J Oral Microbiol. 2019; 11:1565043. https://doi.org/10.1080/20002297.2018.1565043. [Pubmed]
- 67. CEACAMs serve as toxin-stimulated receptors for enterotoxigenic Escherichia coli. Proc Natl Acad Sci U S A. 2020; 117:29055–62. https://doi.org/10.1073/pnas.2012480117. [Pubmed]
- 68. Binding of Escherichia coli and Salmonella strains to members of the carcinoembryonic antigen family: differential binding inhibition by aromatic alpha-glycosides of mannose. Infect Immun. 1991; 59:2051–57. https://doi.org/10.1128/iai.59.6.2051-2057.1991. [Pubmed]
- 69. Helicobacter pylori adhesin HopQ engages in a virulence-enhancing interaction with human CEACAMs. Nat Microbiol. 2016; 2:16189. https://doi.org/10.1038/nmicrobiol.2016.189. [Pubmed]
- 70. Structure and function of Helicobacter pylori CagA, the first-identified bacterial protein involved in human cancer. Proc Jpn Acad Ser B Phys Biol Sci. 2017; 93:196–219. https://doi.org/10.2183/pjab.93.013. [Pubmed]
- 71. The HopQ-CEACAM Interaction Controls CagA Translocation, Phosphorylation, and Phagocytosis of Helicobacter pylori in Neutrophils. mBio. 2020; 11:1–19. https://doi.org/10.1128/mBio.03256-19. [Pubmed]
- 72. Tolerance rather than immunity protects from Helicobacter pylori-induced gastric preneoplasia. Gastroenterology. 2011; 140:199–209. https://doi.org/10.1053/j.gastro.2010.06.047. [Pubmed]
- 73. Functional plasticity in the type IV secretion system of Helicobacter pylori. PLoS Pathog. 2013; 9:e1003189. https://doi.org/10.1371/journal.ppat.1003189. [Pubmed]
- 74. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010; 23:713–39. https://doi.org/10.1128/CMR.00011-10. [Pubmed]
- 75. Bacterial protein domains with a novel Ig-like fold target human CEACAM receptors. EMBO J. 2021; 40:e106103. https://doi.org/10.15252/embj.2020106103. [Pubmed]
- 76. Carcinoembryonic antigen-related cellular adhesion molecule 1 isoforms alternatively inhibit and costimulate human T cell function. J Immunol. 2004; 172:3535–43. https://doi.org/10.4049/jimmunol.172.6.3535. [Pubmed]
- 77. Regulation of human intestinal intraepithelial lymphocyte cytolytic function by biliary glycoprotein (CD66a). J Immunol. 1999; 163:1363–70. [Pubmed]
- 78. EpCAM-high liver cancer stem cells resist natural killer cell-mediated cytotoxicity by upregulating CEACAM1. J Immunother Cancer. 2020; 8:e000301. https://doi.org/10.1136/jitc-2019-000301. [Pubmed]
- 79. Shc and CEACAM1 interact to regulate the mitogenic action of insulin. J Biol Chem. 2002; 277:1076–84. https://doi.org/10.1074/jbc.M108415200. [Pubmed]
- 80. CEACAM1 modulates epidermal growth factor receptor--mediated cell proliferation. J Clin Invest. 2004; 114:944–52. https://doi.org/10.1172/JCI21786. [Pubmed]
- 81. Deletion of the carcinoembryonic antigen-related cell adhesion molecule 1 (Ceacam1) gene contributes to colon tumor progression in a murine model of carcinogenesis. Oncogene. 2006; 25:5527–36. https://doi.org/10.1038/sj.onc.1209541. [Pubmed]
- 82. Loss of CEACAM1 is associated with poor prognosis and peritoneal dissemination of patients with gastric cancer. Sci Rep. 2019; 9:12702. https://doi.org/10.1038/s41598-019-49230-w. [Pubmed]
- 83. Expression of CD66a (human C-CAM) and other members of the carcinoembryonic antigen gene family of adhesion molecules in human colorectal adenomas. Cancer Res. 1997; 57:2354–57. [Pubmed]
- 84. The CEACAM1-mediated apoptosis pathway is activated by CEA and triggers dual cleavage of CEACAM1. Oncogene. 2008; 27:3721–28. https://doi.org/10.1038/sj.onc.1211033. [Pubmed]
- 85. Carcinoembryonic antigen-related cell adhesion molecule 1 inhibits proximal TCR signaling by targeting ZAP-70. J Immunol. 2008; 180:6085–93. https://doi.org/10.4049/jimmunol.180.9.6085. [Pubmed]
- 86. The cell adhesion molecule CEACAM1-L is a substrate of caspase-3-mediated cleavage in apoptotic mouse intestinal cells. J Biol Chem. 2003; 278:16929–35. https://doi.org/10.1074/jbc.M301842200. [Pubmed]
- 87. Identification and quantification of aberrant crypt foci and microadenomas in the human colon. Hum Pathol. 1991; 22:287–94. https://doi.org/10.1016/0046-8177(91)90163-j. [Pubmed]
- 88. Gene expression profiles in normal and cancer cells. Science. 1997; 276:1268–72. https://doi.org/10.1126/science.276.5316.1268. [Pubmed]
- 89. The human tumor suppressor CEACAM1 modulates apoptosis and is implicated in early colorectal tumorigenesis. Oncogene. 2004; 23:9306–13. https://doi.org/10.1038/sj.onc.1208259. [Pubmed]
- 90. Stromal CEACAM1 expression regulates colorectal cancer metastasis. Oncoimmunology. 2012; 1:1205–7. https://doi.org/10.4161/onci.20735. [Pubmed]
- 91. Aging-related carcinoembryonic antigen-related cell adhesion molecule 1 signaling promotes vascular dysfunction. Aging Cell. 2019; 18:e13025. https://doi.org/10.1111/acel.13025. [Pubmed]
- 92. Roles and mechanisms of circulating CEACAM1 in the cirrhosis-related intestinal hyperpermeability: in vitro approach. J Chin Med Assoc. 2021; 84:851–59. https://doi.org/10.1097/JCMA.0000000000000582. [Pubmed]
- 93. CEACAM1 loss links inflammation to insulin resistance in obesity and non-alcoholic steatohepatitis (NASH). Semin Immunopathol. 2014; 36:55–71. https://doi.org/10.1007/s00281-013-0407-3. [Pubmed]
- 94. TGF-β Signaling in Liver, Pancreas, and Gastrointestinal Diseases and Cancer. Gastroenterology. 2021; 161:434–52.e15. https://doi.org/10.1053/j.gastro.2021.04.064. [Pubmed]
- 95. β2-spectrin (SPTBN1) as a therapeutic target for diet-induced liver disease and preventing cancer development. Sci Transl Med. 2021; 13:eabk2267. https://doi.org/10.1126/scitranslmed.abk2267. [Pubmed]
- 96. Carcinoembryonic antigen-related cell adhesion molecule 1a-4L suppression of rat hepatocellular carcinomas. Cancer Res. 2005; 65:11010–17. https://doi.org/10.1158/0008-5472.CAN-04-2841. [Pubmed]
- 97. CEACAM1, a novel serum biomarker for pancreatic cancer. Pancreas. 2007; 34:436–43. https://doi.org/10.1097/MPA.0b013e3180333ae3. [Pubmed]
- 98. Identification of Serum Biomarker Panels for the Early Detection of Pancreatic Cancer. Cancer Epidemiol Biomarkers Prev. 2019; 28:174–82. https://doi.org/10.1158/1055-9965.EPI-18-0483. [Pubmed]
- 99. Carcinoembryonic antigen-related cell adhesion molecules (CEACAM) 1, 5 and 6 as biomarkers in pancreatic cancer. PLoS One. 2014; 9:e113023. https://doi.org/10.1371/journal.pone.0113023. [Pubmed]
- 100. Identification of CEACAM5 as a Biomarker for Prewarning and Prognosis in Gastric Cancer. J Histochem Cytochem. 2015; 63:922–30. https://doi.org/10.1369/0022155415609098. [Pubmed]
- 101. CEACAM5 has different expression patterns in gastric non-neoplastic and neoplastic lesions and cytoplasmic staining is a marker for evaluation of tumor progression in gastric adenocarcinoma. Pathol Res Pract. 2014; 210:686–93. https://doi.org/10.1016/j.prp.2014.06.024. [Pubmed]
- 102. Carcinoembryonic antigen (CEA) inhibits NK killing via interaction with CEA-related cell adhesion molecule 1. J Immunol. 2005; 174:6692–701. https://doi.org/10.4049/jimmunol.174.11.6692. [Pubmed]
- 103. Targeted killing of colorectal cancer cell lines by a humanised IgG1 monoclonal antibody that binds to membrane-bound carcinoembryonic antigen. Br J Cancer. 2008; 98:1217–25. https://doi.org/10.1038/sj.bjc.6604289. [Pubmed]
- 104. Development and Characterization of an Anti-Cancer Monoclonal Antibody for Treatment of Human Carcinomas. Cancers (Basel). 2022; 14:3037. https://doi.org/10.3390/cancers14133037. [Pubmed]
- 105. Carcinoembryonic antigen inhibits anoikis in colorectal carcinoma cells by interfering with TRAIL-R2 (DR5) signaling. Cancer Res. 2007; 67:4774–82. https://doi.org/10.1158/0008-5472.CAN-06-4315. [Pubmed]
- 106. Carcinoembryonic antigen (CEA) and its receptor hnRNP M are mediators of metastasis and the inflammatory response in the liver. Clin Exp Metastasis. 2011; 28:923–32. https://doi.org/10.1007/s10585-011-9419-3. [Pubmed]
- 107. Mutational Profiles Reveal an Aberrant TGF-β-CEA Regulated Pathway in Colon Adenomas. PLoS One. 2016; 11:e0153933. https://doi.org/10.1371/journal.pone.0153933. [Pubmed]
- 108. Carcinoembryonic antigen interacts with TGF-{beta} receptor and inhibits TGF-{beta} signaling in colorectal cancers. Cancer Res. 2010; 70:8159–68. https://doi.org/10.1158/0008-5472.CAN-10-1073. [Pubmed]
- 109. CEACAM5 and CEACAM6 are major target genes for Smad3-mediated TGF-beta signaling. Oncogene. 2008; 27:675–83. https://doi.org/10.1038/sj.onc.1210686. [Pubmed]
- 110. Mutated CEACAMs Disrupt Transforming Growth Factor Beta Signaling and Alter the Intestinal Microbiome to Promote Colorectal Carcinogenesis. Gastroenterology. 2020; 158:238–52. https://doi.org/10.1053/j.gastro.2019.09.023. [Pubmed]
- 111. Interleukin-6 trans-signaling increases the expression of carcinoembryonic antigen-related cell adhesion molecules 5 and 6 in colorectal cancer cells. BMC Cancer. 2015; 15:975. https://doi.org/10.1186/s12885-015-1950-1. [Pubmed]
- 112. Expression patterns of CEACAM5 and CEACAM6 in primary and metastatic cancers. BMC Cancer. 2007; 7:2. https://doi.org/10.1186/1471-2407-7-2. [Pubmed]
- 113. CEACAM6 is a prognostic biomarker and potential therapeutic target for gastric carcinoma. Oncotarget. 2017; 8:83673–83. https://doi.org/10.18632/oncotarget.19415. [Pubmed]
- 114. Overexpression and clinical significance of carcinoembryonic antigen-related cell adhesion molecule 6 in colorectal cancer. Clin Chim Acta. 2013; 415:12–19. https://doi.org/10.1016/j.cca.2012.09.003. [Pubmed]
- 115. Expression and prognostic significance of CEACAM6, ITGB1, and CYR61 in peripheral blood of patients with gastric cancer. J Surg Oncol. 2011; 104:525–29. https://doi.org/10.1002/jso.21984. [Pubmed]
- 116. CEACAM6 induces epithelial-mesenchymal transition and mediates invasion and metastasis in pancreatic cancer. Int J Oncol. 2013; 43:877–85. https://doi.org/10.3892/ijo.2013.2015. [Pubmed]
- 117. Carcinoembryonic Antigen Related Cell Adhesion Molecule 6 Promotes Carcinogenesis of Gastric Cancer and Anti-CEACAM6 Fluorescent Probe Can Diagnose the Precancerous Lesions. Front Oncol. 2021; 11:643669. https://doi.org/10.3389/fonc.2021.643669. [Pubmed]
- 118. CEACAM6 gene silencing impairs anoikis resistance and in vivo metastatic ability of pancreatic adenocarcinoma cells. Oncogene. 2004; 23:465–73. https://doi.org/10.1038/sj.onc.1207036. [Pubmed]
- 119. CEACAM6 cross-linking induces caveolin-1-dependent, Src-mediated focal adhesion kinase phosphorylation in BxPC3 pancreatic adenocarcinoma cells. J Biol Chem. 2004; 279:23176–82. https://doi.org/10.1074/jbc.M402051200. [Pubmed]
- 120. Insulin acutely decreases hepatic fatty acid synthase activity. Cell Metab. 2005; 2:43–53. https://doi.org/10.1016/j.cmet.2005.06.001. [Pubmed]
- 121. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut. 2014; 63:116–24. https://doi.org/10.1136/gutjnl-2012-304119. [Pubmed]
- 122. Thiazolylaminomannosides as potent antiadhesives of type 1 piliated Escherichia coli isolated from Crohn’s disease patients. J Med Chem. 2013; 56:5395–406. https://doi.org/10.1021/jm400723n. [Pubmed]
- 123. CEACAM1 regulates insulin clearance in liver. Nat Genet. 2002; 30:270–76. https://doi.org/10.1038/ng840. [Pubmed]
- 124. Mechanism of glucose intolerance in mice with dominant negative mutation of CEACAM1. Am J Physiol Endocrinol Metab. 2006; 291:E517–24. https://doi.org/10.1152/ajpendo.00077.2006. [Pubmed]
- 125. Potent induction of B- and T-cell immunity against human carcinoembryonic antigen-expressing tumors in human carcinoembryonic antigen transgenic mice mediated by direct lentivector injection. Mol Cancer Ther. 2009; 8:692–702. https://doi.org/10.1158/1535-7163.MCT-08-0769. [Pubmed]
- 126. Preventive DNA vaccination against CEA-expressing tumors with anti-idiotypic scFv6.C4 DNA in CEA-expressing transgenic mice. Cancer Immunol Immunother. 2017; 66:333–42. https://doi.org/10.1007/s00262-016-1940-4. [Pubmed]
- 127. Carcinoembryonic antigen transgenic mouse models for immunotherapy and development of cancer vaccines. Curr Protoc Immunol. 2008; Chapter 20:20.8.1–12. https://doi.org/10.1002/0471142735.im2008s80. [Pubmed]
- 128. Radioimmunoimaging of liver metastases with PET using a 64Cu-labeled CEA antibody in transgenic mice. PLoS One. 2014; 9:e106921. https://doi.org/10.1371/journal.pone.0106921. [Pubmed]
- 129. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat Methods. 2006; 3:793–95. https://doi.org/10.1038/nmeth929. [Pubmed]
- 130. Cryo-EM in drug discovery: achievements, limitations and prospects. Nat Rev Drug Discov. 2018; 17:471–92. https://doi.org/10.1038/nrd.2018.77. [Pubmed]
- 131. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011; 141:1762–72. https://doi.org/10.1053/j.gastro.2011.07.050. [Pubmed]
- 132. Liver Organoids: Recent Developments, Limitations and Potential. Front Med (Lausanne). 2021; 8:574047. https://doi.org/10.3389/fmed.2021.574047. [Pubmed]
- 133. Modeling Steatohepatitis in Humans with Pluripotent Stem Cell-Derived Organoids. Cell Metab. 2019; 30:374–84.e6. https://doi.org/10.1016/j.cmet.2019.05.007. [Pubmed]
- 134. Generation of hepatobiliary organoids from human induced pluripotent stem cells. J Hepatol. 2019; 70:1145–58. https://doi.org/10.1016/j.jhep.2018.12.028. [Pubmed]
- 135. Generation of Hepatic Stellate Cells from Human Pluripotent Stem Cells Enables In Vitro Modeling of Liver Fibrosis. Cell Stem Cell. 2018; 23:101–13.e7. https://doi.org/10.1016/j.stem.2018.05.027. [Pubmed]
- 136. Massive and Reproducible Production of Liver Buds Entirely from Human Pluripotent Stem Cells. Cell Rep. 2017; 21:2661–70. https://doi.org/10.1016/j.celrep.2017.11.005. [Pubmed]
- 137. Targeted disruption of carcinoembryonic antigen-related cell adhesion molecule 1 promotes diet-induced hepatic steatosis and insulin resistance. Endocrinology. 2009; 150:3503–12. https://doi.org/10.1210/en.2008-1439. [Pubmed]
- 138. Mice with null mutation of Ceacam I develop nonalcoholic steatohepatitis. Hepat Med. 2010; 2010:69–78. https://doi.org/10.2147/HMER.S8902. [Pubmed]
- 139. Reversal of obesity development in Ceacam1−/− male mice by bone marrow transplantation or introduction of the human CEACAM1 gene. Obesity (Silver Spring). 2022; 30:1351–56. https://doi.org/10.1002/oby.23457. [Pubmed]
- 140. Adherent-invasive Escherichia coli induce claudin-2 expression and barrier defect in CEABAC10 mice and Crohn’s disease patients. Inflamm Bowel Dis. 2012; 18:294–304. https://doi.org/10.1002/ibd.21787. [Pubmed]
- 141. Increased colon tumor susceptibility in azoxymethane treated CEABAC transgenic mice. Carcinogenesis. 2006; 27:1909–16. https://doi.org/10.1093/carcin/bgl040. [Pubmed]
- 142. [The HopQ-CEACAM interaction controls CagA translocation, phosphorylation, and phagocytosis of Helicobacter pylori in neutrophilic granulocytes]. Med Sci (Paris). 2021; 37:403–5. https://doi.org/10.1051/medsci/2021042. [Pubmed]
